Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain?
- PMID: 20456524
- PMCID: PMC2913007
- DOI: 10.1111/j.1469-7580.2010.01227.x
Intervertebral disc, sensory nerves and neurotrophins: who is who in discogenic pain?
Abstract
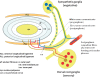
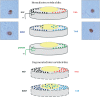
The normal intervertebral disc (IVD) is a poorly innervated organ supplied only by sensory (mainly nociceptive) and postganglionic sympathetic (vasomotor efferents) nerve fibers. Interestingly, upon degeneration, the IVD becomes densely innervated even in regions that in normal conditions lack innervation. This increased innervation has been associated with pain of IVD origin. The mechanisms responsible for nerve growth and hyperinnervation of pathological IVDs have not been fully elucidated. Among the molecules that are presumably involved in this process are some members of the family of neurotrophins (NTs), which are known to have both neurotrophic and neurotropic properties and regulate the density and distribution of nerve fibers in peripheral tissues. NTs and their receptors are expressed in healthy IVDs but much higher levels have been observed in pathological IVDs, thus suggesting a correlation between levels of expression of NTs and density of innervation in IVDs. In addition, NTs also play a role in inflammatory responses and pain transmission by increasing the expression of pain-related peptides and modulating synapses of nociceptive neurons at the spinal cord. This article reviews current knowledge about the innervation of IVDs, NTs and NT receptors, expression of NTs and their receptors in IVDs as well as in the sensory neurons innervating the IVDs, the proinflammatory role of NTs, NTs as nociception regulators, and the potential network of discogenic pain involving NTs.
Figures




References
-
- Abe Y, Akeda K, An HS, et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–642. - PubMed
-
- Acheson A, Conover JC, Fandl JP, et al. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. - PubMed
-
- Ahn SH, Cho YW, Ahn MW, et al. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. - PubMed
-
- Aoki Y, Ohtori S, Takahashi K, et al. Innervation of the lumbar intervertebral disc by nerve growth factor-dependent neurons related to inflammatory pain. Spine. 2004a;29:1077–1081. - PubMed
-
- Aoki Y, Takahashi Y, Takahashi K, et al. Sensory innervation of the lateral portion of the lumbar intervertebral discs in rats. Spine J. 2004b;4:275–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

