Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells
- PMID: 20456695
- PMCID: PMC2974804
- DOI: 10.1111/j.1537-2995.2010.02664.x
Complement C1q enhances homing-related responses of hematopoietic stem/progenitor cells
Abstract
Background: Previously, we reported that the complement cleavage fragments C3a and C5a are important modulators of trafficking of hematopoietic stem/progenitor cells (HSPCs). The aim of this study was to examine a possible role for complement component 1, subcomponent q (C1q) in HSPC migration.
Study design and methods: CD34+ HSPCs isolated from cord blood (CB), bone marrow (BM), and granulocyte-colony-stimulating factor (G-CSF)-mobilized peripheral blood (mPB) were evaluated for the expression of C1q and its receptor for phagocytosis (C1qRp) using reverse transcription-polymerase chain reaction, Western blotting, and fluorescence-activated cell sorting. Chemotactic responses and chemoinvasiveness toward stromal cell-derived factor (SDF)-1 and expression of matrix metalloproteinase (MMP)-9 were also examined after C1q stimulation. Moreover, G-CSF- and zymosan-induced mobilization was evaluated in C1q-deficient mice.
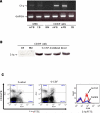
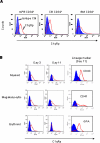
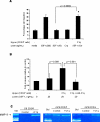
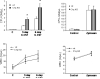
Results: C1q was expressed in CD34+ cells from mPB, but not from CB or steady-state BM; however, stimulation of the latter with G-CSF induced C1q expression. C1qRp receptor was found on BM, CB, and mPB CD34+ cells and more mature ex vivo expanded myeloid and megakaryocytic precursors. Although C1q itself was not a chemoattractant for HSPCs, it primed/enhanced the chemotactic response of CD34+ cells to a low SDF-1 gradient and their chemoinvasion across the reconstituted basement membrane Matrigel and increased secretion of MMP-9 by these cells. Moreover, in in vivo studies C1q-deficient mice were found to be easy G-CSF mobilizers compared to wild-type mice and normal zymosan mobilizers.
Conclusion: We demonstrated that C1q primes the responses of CD34+ HSPCs to an SDF-1 gradient, which may enhance their ability to stay within BM niches, suggesting that the C1q/C1qRp axis contributes to HSPC homing/retention in BM.
© 2010 American Association of Blood Banks.
Figures
Similar articles
-
The HGF/c-Met axis synergizes with G-CSF in the mobilization of hematopoietic stem/progenitor cells.Stem Cells Dev. 2010 Aug;19(8):1143-51. doi: 10.1089/scd.2009.0376. Stem Cells Dev. 2010. PMID: 20021260
-
Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells.Exp Hematol. 2010 Apr;38(4):321-32. doi: 10.1016/j.exphem.2010.02.002. Epub 2010 Feb 12. Exp Hematol. 2010. PMID: 20153802 Free PMC article.
-
Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient.Blood. 2005 Jan 1;105(1):40-8. doi: 10.1182/blood-2004-04-1430. Epub 2004 Aug 24. Blood. 2005. PMID: 15328152
-
A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC).Adv Exp Med Biol. 2008;632:47-60. Adv Exp Med Biol. 2008. PMID: 19025113 Review.
-
The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells.Cytokines Mol Ther. 1995 Dec;1(4):249-70. Cytokines Mol Ther. 1995. PMID: 9384679 Review.
Cited by
-
Cationic liposome-mediated CXCR4 gene delivery into hematopoietic stem/progenitor cells: implications for clinical transplantation and gene therapy.Stem Cells Dev. 2012 Jul 1;21(10):1587-96. doi: 10.1089/scd.2011.0297. Epub 2011 Dec 14. Stem Cells Dev. 2012. PMID: 22047530 Free PMC article.
-
The role of complement in the trafficking of hematopoietic stem/progenitor cells.Transfusion. 2012 Dec;52(12):2706-16. doi: 10.1111/j.1537-2995.2012.03636.x. Epub 2012 Apr 9. Transfusion. 2012. PMID: 22486360 Free PMC article. Review. No abstract available.
-
Low Molecular Weight Fraction of Commercial Human Serum Albumin Induces Morphologic and Transcriptional Changes of Bone Marrow-Derived Mesenchymal Stem Cells.Stem Cells Transl Med. 2015 Aug;4(8):945-55. doi: 10.5966/sctm.2014-0293. Epub 2015 Jun 3. Stem Cells Transl Med. 2015. PMID: 26041739 Free PMC article.
-
Innate immunity orchestrates the mobilization and homing of hematopoietic stem/progenitor cells by engaging purinergic signaling-an update.Purinergic Signal. 2020 Jun;16(2):153-166. doi: 10.1007/s11302-020-09698-y. Epub 2020 May 15. Purinergic Signal. 2020. PMID: 32415576 Free PMC article. Review.
-
The ins and outs of hematopoietic stem cells: studies to improve transplantation outcomes.Stem Cell Rev Rep. 2011 Sep;7(3):590-607. doi: 10.1007/s12015-010-9212-8. Stem Cell Rev Rep. 2011. PMID: 21140298 Free PMC article. Review.
References
-
- Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–98. - PubMed
-
- Lu J, Wu X. The BK. The regulatory roles of C1q. Immunobiol. 2007;212:245–52. - PubMed
-
- Sjoberg AP, Trouw LA, Blom AM. Complement activation and inhibition: a delicate balance. Trends Immunol. 2009;30:83–90. - PubMed
-
- Kishore U, Reid KBM. C1q: structure, function, and receptors. Immunopharmacol. 2000;49:159–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

