Platelet senescence and phosphatidylserine exposure
- PMID: 20456701
- PMCID: PMC2921562
- DOI: 10.1111/j.1537-2995.2010.02676.x
Platelet senescence and phosphatidylserine exposure
Abstract
Background: The exposure of phosphatidylserine occurs during platelet (PLT) activation and during in vitro storage. Phosphatidylserine exposure also occurs during apoptosis after the release of mitochondrial cytochrome c. We have examined the role of cytochrome c release, mitochondrial membrane potential (ΔΨm), and cyclophilin D (CypD) in phosphatidylserine exposure due to activation and storage.
Study design and methods: The exposure of phosphatidylserine and the loss of ΔΨm were determined in a flow cytometer using fluorescein isothiocyanate-lactadherin and JC-1, a lipophilic cationic reporter dye. The role of CypD was determined with cyclosporin A and CypD-deficient murine PLTs. Cytochrome c-induced caspase-3 and Rho-associated kinase I (ROCK1) activation were determined by immunoblotting and using their inhibitors.
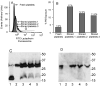
Results: Collagen- and thrombin-induced exposure of phosphatidylserine was accompanied by a decrease in ΔΨm. Cyclosporin A inhibited the phosphatidylserine exposure and the loss of ΔΨm. CypD(-/-) mice had decreased loss of ΔΨm and impaired phosphatidylserine exposure. Collagen and thrombin did not induce the release of cytochrome c nor the activation of caspase-3 and ROCK1. In contrast, in PLTs stored for more than 5 days, the phosphatidylserine exposure was associated with cytochrome c-induced caspase-3 and ROCK1 activation. ABT737, a BH3 mimetic that induces mitochondrial pathway of apoptosis, induced cytochrome c release and activation of caspase-3 and ROCK1 and phosphatidylserine exposure independent of CypD.
Conclusion: These results show that in stored PLTs cytochrome c release and the subsequent activation of caspase-3 and ROCK1 mediate phosphatidylserine exposure and it is distinct from activation-induced phosphatidylserine exposure.
© 2010 American Association of Blood Banks.
Conflict of interest statement
Conflict-of-interest: The authors declare no competing financial interest.
Figures

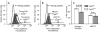



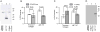
References
-
- Comfurius P, Senden JM, Tilly RH, Schroit AJ, Bevers EM, Zwaal RF. Loss of membrane phospholipid asymmetry in platelets and red cells may be associated with calcium-induced shedding of plasma membrane and inhibition of aminophospholipid translocase. Biochim Biophys Acta. 1990;1026:153–160. - PubMed
-
- Thiagarajan P, Tait JF. Collagen-induced exposure of anionic phospholipid in platelets and platelet-derived microparticles. J Biol Chem. 1991;266:24302–24307. - PubMed
-
- Solum NO. Procoagulant expression in platelets and defects leading to clinical disorders. Arterioscler Thromb Vasc Biol. 1999;19:2841–2846. - PubMed
-
- Ahmad SS, Rawala-Sheikh R, Walsh PN. Components and assembly of the factor X activating complex. Semin Thromb Hemost. 1992;18:311–323. - PubMed
-
- Bratosin D, Mazurier J, Tissier JP, Estaquier J, Huart JJ, Ameisen JC, Aminoff D, Montreuil J. Cellular and molecular mechanisms of senescent erythrocyte phagocytosis by macrophages. A review. Biochimie. 1998;80:173–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

