Trigenic neural crest-restricted Smad7 over-expression results in congenital craniofacial and cardiovascular defects
- PMID: 20457144
- PMCID: PMC2909335
- DOI: 10.1016/j.ydbio.2010.05.004
Trigenic neural crest-restricted Smad7 over-expression results in congenital craniofacial and cardiovascular defects
Abstract
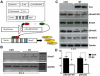
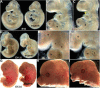
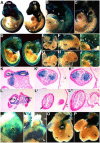
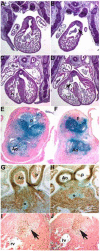
Smad7 is a negative regulator of TGFbeta superfamily signaling. Using a three-component triple transgenic system, expression of the inhibitory Smad7 was induced via doxycycline within the NCC lineages at pre- and post-migratory stages. Consistent with its role in negatively regulating both TGFbeta and BMP signaling in vitro, induction of Smad7 within the NCC significantly suppressed phosphorylation levels of both Smad1/5/8 and Smad2/3 in vivo, resulting in subsequent loss of NCC-derived craniofacial, pharyngeal and cardiac OFT cushion cells. At the cellular level, increased cell death was observed in pharyngeal arches. However, cell proliferation and NCC-derived smooth muscle differentiation were unaltered. NCC lineage mapping demonstrated that cardiac NCC emigration and initial migration were not affected, but subsequent colonization of the OFT was significantly reduced. Induction of Smad7 in post-migratory NCC resulted in interventricular septal chamber septation defects, suggesting that TGFbeta superfamily signaling is also essential for cardiac NCC at post-migratory stages to govern normal cardiac development. Taken together, the data illustrate that tightly regulated TGFbeta superfamily signaling plays an essential role during craniofacial and cardiac NCC colonization and cell survival in vivo.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Loss of microRNAs in neural crest leads to cardiovascular syndromes resembling human congenital heart defects.Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2575-86. doi: 10.1161/ATVBAHA.110.213306. Epub 2010 Sep 30. Arterioscler Thromb Vasc Biol. 2010. PMID: 20884876 Free PMC article.
-
Neural crest cell-specific deletion of Rac1 results in defective cell-matrix interactions and severe craniofacial and cardiovascular malformations.Dev Biol. 2010 Apr 15;340(2):613-25. doi: 10.1016/j.ydbio.2010.02.021. Epub 2010 Feb 23. Dev Biol. 2010. PMID: 20184871 Free PMC article.
-
mTOR acts as a pivotal signaling hub for neural crest cells during craniofacial development.PLoS Genet. 2018 Jul 5;14(7):e1007491. doi: 10.1371/journal.pgen.1007491. eCollection 2018 Jul. PLoS Genet. 2018. PMID: 29975682 Free PMC article.
-
Neural crest cells in cardiovascular development.Curr Top Dev Biol. 2015;111:183-200. doi: 10.1016/bs.ctdb.2014.11.006. Epub 2015 Jan 20. Curr Top Dev Biol. 2015. PMID: 25662261 Review.
-
TGFβ superfamily signaling in the neural crest lineage.Cell Adh Migr. 2011 May-Jun;5(3):232-6. doi: 10.4161/cam.5.3.15498. Epub 2011 May 1. Cell Adh Migr. 2011. PMID: 21436616 Free PMC article. Review.
Cited by
-
Dullard-mediated Smad1/5/8 inhibition controls mouse cardiac neural crest cells condensation and outflow tract septation.Elife. 2020 Feb 27;9:e50325. doi: 10.7554/eLife.50325. Elife. 2020. PMID: 32105214 Free PMC article.
-
Pax3 is essential for normal cardiac neural crest morphogenesis but is not required during migration nor outflow tract septation.Dev Biol. 2011 Aug 15;356(2):308-22. doi: 10.1016/j.ydbio.2011.05.583. Epub 2011 May 12. Dev Biol. 2011. PMID: 21600894 Free PMC article.
-
RUNX3, EGR1 and SOX9B form a regulatory cascade required to modulate BMP-signaling during cranial cartilage development in zebrafish.PLoS One. 2012;7(11):e50140. doi: 10.1371/journal.pone.0050140. Epub 2012 Nov 27. PLoS One. 2012. PMID: 23209659 Free PMC article.
-
Association of two variants in SMAD7 with the risk of congenital heart disease in the Han Chinese population.PLoS One. 2013 Sep 5;8(9):e72423. doi: 10.1371/journal.pone.0072423. eCollection 2013. PLoS One. 2013. PMID: 24039762 Free PMC article.
-
Probing human cardiovascular congenital disease using transgenic mouse models.Prog Mol Biol Transl Sci. 2011;100:83-110. doi: 10.1016/B978-0-12-384878-9.00003-0. Prog Mol Biol Transl Sci. 2011. PMID: 21377625 Free PMC article. Review.
References
-
- Arnold NB, Ketterer K, Kleeff J, Friess H, Büchler MW, Korc M. Thioredoxin is downstream of Smad7 in a pathway that promotes growth and suppresses cisplatin-induced apoptosis in pancreatic cancer. Cancer Res. 2004;64(10):3599–606. - PubMed
-
- Aybar MJ, Mayor R. Early induction of neural crest cells: lessons learned from frog, fish and chick. Curr Opin Genet Dev. 2002;12(4):452–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

