Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft
- PMID: 20457189
- PMCID: PMC2942017
- DOI: 10.1016/j.jconrel.2010.05.005
Correlation of tissue drug concentrations with in vivo magnetic resonance images of polymer drug depot around arteriovenous graft
Abstract
Sustained delivery of anti-proliferative drugs to the perivascular area using an injectable polymeric platform is a strategy to inhibit vascular hyperplasia and stenosis. In this study, the concentrations of sirolimus in vascular tissues were evaluated after delivery using an injectable platform made of poly(lactic-co-glycolic acid)-polyethylene glycol-poly(lactic-co-glycolic acid) (PLGA-PEG-PLGA). In order to optimize the drug release profile, the effect of two solvents or solid loading of the sirolimus into the polymer gel was first examined in vitro. The early release was slower with loading of dry drug into the polymer, compared to drug dissolution in solvents. Dry sirolimus was therefore used to load the polymer and applied to the perivascular surface of the graft-venous anastomosis at the time of surgical placement of a carotid-jugular synthetic hemodialysis graft in a porcine model. This was replenished by ultrasound-guided injection of additional drug-laden polymer at one, two and three weeks post-operatively. Magnetic resonance imaging (MRI) using pulse sequences specifically designed for optimal detection of the polymeric gel showed that the polymer injected post-operatively remained at the juxta-anastomotic perivascular site at two weeks. Sirolimus was extracted from various segments of the juxta-anastomotic tissues and the drug concentrations were determined using HPLC MS/MS. Tissue sirolimus concentrations at one and two weeks were highest near the venous anastomosis, which were approximately 100- to 500-fold greater than the concentrations necessary to inhibit vascular smooth muscle cell proliferation in vitro. Drug concentrations remained above the inhibitory concentrations for at least six weeks post-operatively. Thus, serial injections of sustained-delivery polymer gel loaded with sirolimus can provide high localized concentrations at target vascular tissues and thus may be useful for the prevention and treatment of vascular proliferative disorders such as hemodialysis graft stenosis. In addition, MRI is useful for the monitoring of the location of the drug depot.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures




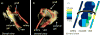
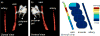
Similar articles
-
A biodegradable perivascular wrap for controlled, local and directed drug delivery.J Control Release. 2012 Jul 10;161(1):81-9. doi: 10.1016/j.jconrel.2012.04.029. Epub 2012 Apr 27. J Control Release. 2012. PMID: 22561340 Free PMC article.
-
In vivo evaluation of the delivery and efficacy of a sirolimus-laden polymer gel for inhibition of hyperplasia in a porcine model of arteriovenous hemodialysis graft stenosis.J Control Release. 2012 Jun 28;160(3):459-67. doi: 10.1016/j.jconrel.2012.03.011. Epub 2012 Mar 17. J Control Release. 2012. PMID: 22465391 Free PMC article.
-
Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rabbit vein graft model.J Vasc Surg. 2018 Dec;68(6S):188S-200S.e4. doi: 10.1016/j.jvs.2018.05.206. Epub 2018 Jul 29. J Vasc Surg. 2018. PMID: 30064835 Free PMC article.
-
Rapamycin-loaded nanoparticles for inhibition of neointimal hyperplasia in experimental vein grafts.Ann Vasc Surg. 2011 May;25(4):538-46. doi: 10.1016/j.avsg.2011.01.003. Ann Vasc Surg. 2011. PMID: 21549923
-
Pharmacokinetics and disposition of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) nanoparticles.Curr Drug Metab. 2010 Dec;11(10):859-69. doi: 10.2174/138920010794479682. Curr Drug Metab. 2010. PMID: 21208172 Review.
Cited by
-
A biodegradable perivascular wrap for controlled, local and directed drug delivery.J Control Release. 2012 Jul 10;161(1):81-9. doi: 10.1016/j.jconrel.2012.04.029. Epub 2012 Apr 27. J Control Release. 2012. PMID: 22561340 Free PMC article.
-
Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery.J Control Release. 2016 Jul 10;233:174-80. doi: 10.1016/j.jconrel.2016.05.002. Epub 2016 May 12. J Control Release. 2016. PMID: 27179635 Free PMC article. Review.
-
A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia.J Control Release. 2014 Oct 10;191:47-53. doi: 10.1016/j.jconrel.2014.05.017. Epub 2014 May 20. J Control Release. 2014. PMID: 24852098 Free PMC article.
-
Autologous fat transplants to deliver glitazone and adiponectin for vasculoprotection.J Control Release. 2017 Oct 28;264:237-246. doi: 10.1016/j.jconrel.2017.08.036. Epub 2017 Sep 1. J Control Release. 2017. PMID: 28867378 Free PMC article.
-
A novel Nanocellulose-Gelatin-AS-IV external stent resists EndMT by activating autophagy to prevent restenosis of grafts.Bioact Mater. 2022 Oct 25;22:466-481. doi: 10.1016/j.bioactmat.2022.10.013. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36330163 Free PMC article.
References
-
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002;54(1):3–12. - PubMed
-
- Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54(1):37–51. - PubMed
-
- He C, Kim SW, Lee DS. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J Control Release. 2008;127(3):189–207. - PubMed
-
- Zentner GM, Rathi R, Shih C, McRea JC, Seo MH, Oh H, Rhee BG, Mestecky J, Moldoveanu Z, Morgan M, Weitman S. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J Control Release. 2001;72(1–3):203–215. - PubMed
-
- Uchegbu IF, Schätzlein AG. Polymers in drug delivery. CRC/Taylor & Francis; Boca Raton, FL: 2006. p. 259.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

