CD154 expression is associated with neutralizing antibody titer levels postinfluenza vaccination in stem cell transplant patients and healthy adults
- PMID: 20457264
- PMCID: PMC2933414
- DOI: 10.1016/j.bbmt.2010.04.015
CD154 expression is associated with neutralizing antibody titer levels postinfluenza vaccination in stem cell transplant patients and healthy adults
Abstract
We undertook a prospective longitudinal study to examine humoral and cellular immune responses to influenza vaccination in hematopoietic cell transplant (HCT) patients and healthy adults. Healthy volunteers and HCT patients had blood samples taken prior to influenza vaccination and 30, 90, and 180 days postvaccination. Serum from pre- and postvaccination time points were tested for influenza A IgG and IgM by ELISA as well as tested for neutralizing antibody (NAb) titers via hemagglutination inhibition assay. Polychromatic flow cytometry was used to examine CD4(+) T cells for levels of interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and CD154 (CD40 ligand) expression after stimulation with inactivated flu virus. In healthy subjects, we found a significant increase in Influenza A IgG and IgM levels as well as an increase in NAb titers pre- and post-influenza vaccination. Notably, NAb titers of most HCT patients did not rise to a protective level postvaccination. CD4(+) T cell expression of CD154 and cytokine responses were significantly reduced in HCT recipients compared to healthy adults. A lack of B cell reconstitution and dysfunctional CD4 T cell costimulation (as marked by low CD154 expression) is associated with low NAb levels postvaccination in HCT patients.
Copyright © 2011 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors do not have a commercial or other association that might pose a conflict of interest
Figures



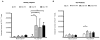
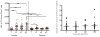
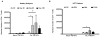
References
-
- Parkman R, Weinberg KI. Immunological reconstitution following bone marrow transplantation. Immunol Rev. 1997;157:73–78. - PubMed
-
- Ljungman P, Lewensohn-Fuchs I, Hammarstrom V, et al. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood. 1994;84:657–663. - PubMed
-
- Blume KG, Forman Stephen J, Appelbaum Frederick R. Thomas’ hematopoietic cell transplantation. Malden, MA: Blackwell Pub; 2004.
-
- Holmberg LA, Boeckh M, Hooper H, et al. Increased incidence of cytomegalovirus disease after autologous CD34-selected peripheral blood stem cell transplantation. Blood. 1999;94:4029–4035. - PubMed
-
- Crippa F, Holmberg L, Carter RA, et al. Infectious complications after autologous CD34-selected peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2002;8:281–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

