Membrane domains based on ankyrin and spectrin associated with cell-cell interactions
- PMID: 20457566
- PMCID: PMC2882121
- DOI: 10.1101/cshperspect.a003012
Membrane domains based on ankyrin and spectrin associated with cell-cell interactions
Abstract
Nodes of Ranvier and axon initial segments of myelinated nerves, sites of cell-cell contact in early embryos and epithelial cells, and neuromuscular junctions of skeletal muscle all perform physiological functions that depend on clustering of functionally related but structurally diverse ion transporters and cell adhesion molecules within microdomains of the plasma membrane. These specialized cell surface domains appeared at different times in metazoan evolution, involve a variety of cell types, and are populated by distinct membrane-spanning proteins. Nevertheless, recent work has shown that these domains all share on their cytoplasmic surfaces a membrane skeleton comprised of members of the ankyrin and spectrin families. This review will summarize basic features of ankyrins and spectrins, and will discuss emerging evidence that these proteins are key players in a conserved mechanism responsible for assembly and maintenance of physiologically important domains on the surfaces of diverse cells.
Figures

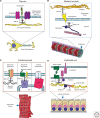

References
-
- Abdi KM, Mohler PJ, Davis JQ, Bennett V 2006. Isoform specificity of ankyrin-B: A site in the divergent C-terminal domain is required for intramolecular association. J Biol Chem 281:5741–5749 - PubMed
-
- An X, Guo X, Sum H, Morrow J, Gratzer W, Mohandas N 2004. Phosphatidylserine binding sites in erythroid spectrin: Location and implications for membrane stability. Biochemistry 43:310–315 - PubMed
-
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ 2004. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell 119:257–272 - PubMed
-
- Ayalon G, Davis JQ, Scotland PB, Bennett V 2008. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135:1189–1200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
