The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway
- PMID: 20457600
- PMCID: PMC2906328
- DOI: 10.1074/jbc.M109.075143
The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway
Abstract
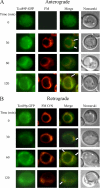
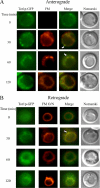
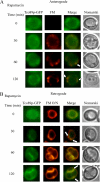
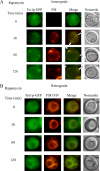
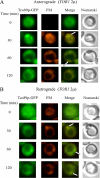
The key gluconeogenic enzyme fructose-1,6-bisphosphatase (FBPase) is induced when Saccharomyces cerevisiae are starved of glucose. However, when glucose is added to cells that have been starved for 3 days, FBPase is degraded in the vacuole. FBPase is first imported to Vid (vacuole import and degradation) vesicles, and these vesicles then merge with the endocytic pathway. In this report we show that two additional gluconeogenic enzymes, isocitrate lyase and phosphoenolpyruvate carboxykinase, were also degraded in the vacuole via the Vid pathway. These new cargo proteins and FBPase interacted with the TORC1 complex during glucose starvation. However, Tor1p was dissociated from FBPase after the addition of glucose. FBPase degradation was inhibited in cells overexpressing TOR1, suggesting that excessive Tor1p is inhibitory. Both Tco89p and Tor1p were found in endosomes coming from the plasma membrane as well as in retrograde vesicles forming from the vacuole membrane. When TORC1 was inactivated by rapamycin, FBPase degradation was inhibited. We suggest that TORC1 interacts with multiple cargo proteins destined for the Vid pathway and plays an important role in the degradation of FBPase in the vacuole.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

