CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes
- PMID: 20457601
- PMCID: PMC2919091
- DOI: 10.1074/jbc.M109.087239
CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes
Abstract
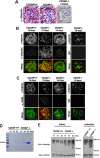
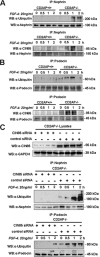
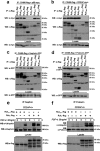
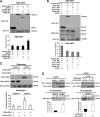
Podocyte damage is the basis of many glomerular diseases with ultrastructural changes and decreased expression of components of the slit diaphragm such as nephrin and podocin. Under physiological conditions it is likely that the slit diaphragm underlies permanent renewal processes to indemnify its stability in response to changes in filtration pressure. This would require constant reorganization of the podocyte foot process and the renewal of slit diaphragm components. Thus far, the mechanisms underlying the turnover of slit diaphragm proteins are largely unknown. In this manuscript we examined a mechanism of nephrin endocytosis via CIN85/Ruk(L)-mediated ubiquitination. We can demonstrate that the loss of nephrin expression and onset of the proteinuria in CD2AP(-/-) mice correlates with an increased accumulation of ubiquitinated proteins and expression of CIN85/Ruk(L) in podocytes. In cultured murine podocytes CD2AP deficiency leads to an early ubiquitination of nephrin and podocin after stimulation with fibroblast growth factor-4. Binding assays with different CIN85/Ruk isoforms and mutants showed that nephrin and podocin are binding to the coiled-coil domain of CIN85/Ruk(L). We found that in the presence of CIN85/Ruk(L), which is involved in down-regulation of receptor-tyrosine kinases, nephrin is internalized after stimulation with fibroblast growth factor-4. Interestingly, coexpression of CIN85/Ruk(L) with CD2AP led to a decreased binding of CIN85/Ruk(L) to nephrin and podocin, which indicates a functional competition between CD2AP and CIN85/Ruk(L). Our results support a novel role for CIN85/Ruk(L) in slit diaphragm turnover and proteinuria.
Figures

Similar articles
-
CIN85 Deficiency Prevents Nephrin Endocytosis and Proteinuria in Diabetes.Diabetes. 2016 Dec;65(12):3667-3679. doi: 10.2337/db16-0081. Epub 2016 Aug 16. Diabetes. 2016. PMID: 27531950 Free PMC article.
-
CD2AP regulates SUMOylation of CIN85 in podocytes.Mol Cell Biol. 2012 Mar;32(6):1068-79. doi: 10.1128/MCB.06106-11. Epub 2011 Dec 27. Mol Cell Biol. 2012. PMID: 22203040 Free PMC article.
-
CD2AP/CIN85 balance determines receptor tyrosine kinase signaling response in podocytes.J Biol Chem. 2007 Mar 9;282(10):7457-64. doi: 10.1074/jbc.M608519200. Epub 2007 Jan 9. J Biol Chem. 2007. PMID: 17213204
-
Slit diaphragm dysfunction in proteinuric states: identification of novel therapeutic targets for nephrotic syndrome.Clin Exp Nephrol. 2009 Aug;13(4):275-280. doi: 10.1007/s10157-009-0162-x. Epub 2009 Mar 7. Clin Exp Nephrol. 2009. PMID: 19266252 Review.
-
CD2-associated protein and glomerular disease.Lancet. 2003 Nov 22;362(9397):1746-8. doi: 10.1016/S0140-6736(03)14856-8. Lancet. 2003. PMID: 14643126 Review.
Cited by
-
The Life of a Kidney Podocyte.Acta Physiol (Oxf). 2025 Aug;241(8):e70081. doi: 10.1111/apha.70081. Acta Physiol (Oxf). 2025. PMID: 40698593 Free PMC article. Review.
-
MicroRNA-29a promotion of nephrin acetylation ameliorates hyperglycemia-induced podocyte dysfunction.J Am Soc Nephrol. 2014 Aug;25(8):1698-709. doi: 10.1681/ASN.2013050527. Epub 2014 Feb 27. J Am Soc Nephrol. 2014. PMID: 24578127 Free PMC article.
-
Membrane trafficking in podocyte health and disease.Pediatr Nephrol. 2013 Sep;28(9):1723-37. doi: 10.1007/s00467-012-2281-y. Epub 2012 Aug 30. Pediatr Nephrol. 2013. PMID: 22932996 Free PMC article. Review.
-
aPKCλ maintains the integrity of the glomerular slit diaphragm through trafficking of nephrin to the cell surface.J Biochem. 2014 Aug;156(2):115-28. doi: 10.1093/jb/mvu022. Epub 2014 Apr 3. J Biochem. 2014. PMID: 24700503 Free PMC article.
-
JNK is antagonized to ensure the correct number of interommatidial cells pattern the Drosophila retina.Dev Biol. 2018 Jan 1;433(1):94-107. doi: 10.1016/j.ydbio.2017.11.002. Epub 2017 Nov 11. Dev Biol. 2018. PMID: 29133184 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

