Temperature-dependent regulation of mycolic acid cyclopropanation in saprophytic mycobacteria: role of the Mycobacterium smegmatis 1351 gene (MSMEG_1351) in CIS-cyclopropanation of alpha-mycolates
- PMID: 20457615
- PMCID: PMC2898377
- DOI: 10.1074/jbc.M110.125724
Temperature-dependent regulation of mycolic acid cyclopropanation in saprophytic mycobacteria: role of the Mycobacterium smegmatis 1351 gene (MSMEG_1351) in CIS-cyclopropanation of alpha-mycolates
Abstract
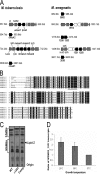
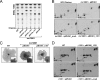
The cell envelope is a crucial determinant of virulence and drug resistance in Mycobacterium tuberculosis. Several features of pathogenesis and immunomodulation of host responses are attributable to the structural diversity in cell wall lipids, particularly in the mycolic acids. Structural modification of the alpha-mycolic acid by introduction of cyclopropane rings as catalyzed by the methyltransferase, PcaA, is essential for a lethal, persistent infection and the cording phenotype in M. tuberculosis. Here, we demonstrate the presence of cyclopropanated cell wall mycolates in the nonpathogenic strain Mycobacterium smegmatis and identify MSMEG_1351 as a gene encoding a PcaA homologue. Interestingly, alpha-mycolic acid cyclopropanation was inducible in cultures grown at 25 degrees C. The growth temperature modulation of the cyclopropanating activity was determined by high resolution magic angle spinning NMR analyses on whole cells. In parallel, quantitative reverse transcription-PCR analysis showed that MSMEG_1351 gene expression is up-regulated at 25 degrees C compared with 37 degrees C. An MSMEG_1351 knock-out strain of M. smegmatis, generated by recombineering, exhibited a deficiency in cyclopropanation of alpha-mycolates. The functional equivalence of PcaA and MSMEG_1351 was established by cross-complementation in the MSMEG_1351 knock-out mutant and also in a DeltapcaA strain of Mycobacterium bovis BCG. Overexpression of MSMEG_1351 restored the wild-type mycolic acid profile and the cording phenotype in BCG. Although the biological significance of mycolic acid cyclopropanation in nonpathogenic mycobacteria remains unclear, it likely represents a mechanism of adaptation of cell wall structure and composition to cope with environmental factors.
Figures


Similar articles
-
Phosphorylation of mycobacterial PcaA inhibits mycolic acid cyclopropanation: consequences for intracellular survival and for phagosome maturation block.J Biol Chem. 2012 Jul 27;287(31):26187-99. doi: 10.1074/jbc.M112.373209. Epub 2012 May 23. J Biol Chem. 2012. PMID: 22621931 Free PMC article.
-
Thiacetazone, an antitubercular drug that inhibits cyclopropanation of cell wall mycolic acids in mycobacteria.PLoS One. 2007 Dec 19;2(12):e1343. doi: 10.1371/journal.pone.0001343. PLoS One. 2007. PMID: 18094751 Free PMC article.
-
Cyclopropanation of α-mycolic acids is not required for cording in Mycobacterium brumae and Mycobacterium fallax.Microbiology (Reading). 2012 Jun;158(Pt 6):1615-1621. doi: 10.1099/mic.0.057919-0. Epub 2012 Apr 5. Microbiology (Reading). 2012. PMID: 22493302
-
[Mycolic acids--biological role and potential application in Mycobacterium detection and differentiation].Postepy Hig Med Dosw (Online). 2014 Apr 4;68:350-8. doi: 10.5604/17322693.1097425. Postepy Hig Med Dosw (Online). 2014. PMID: 24864086 Review. Polish.
-
Mycobacterium Lrp/AsnC family transcriptional factor modulates the arginase pathway as both a sensor and a transcriptional repressor.J Genet Genomics. 2021 Nov 20;48(11):1020-1031. doi: 10.1016/j.jgg.2021.06.018. Epub 2021 Jul 29. J Genet Genomics. 2021. PMID: 34696992 Review.
Cited by
-
Growth of Mycobacterium tuberculosis biofilms.J Vis Exp. 2012 Feb 15;(60):3820. doi: 10.3791/3820. J Vis Exp. 2012. PMID: 22371116 Free PMC article.
-
Dynamic Transcriptional Landscape of Mycobacterium smegmatis under Cold Stress.Int J Mol Sci. 2023 Aug 11;24(16):12706. doi: 10.3390/ijms241612706. Int J Mol Sci. 2023. PMID: 37628885 Free PMC article.
-
Biophysical Interaction Landscape of Mycobacterial Mycolic Acids and Phenolic Glycolipids with Host Macrophage Membranes.ACS Appl Bio Mater. 2023 Dec 18;6(12):5555-5562. doi: 10.1021/acsabm.3c00748. Epub 2023 Nov 28. ACS Appl Bio Mater. 2023. PMID: 38015441 Free PMC article.
-
Phosphorylation of mycobacterial PcaA inhibits mycolic acid cyclopropanation: consequences for intracellular survival and for phagosome maturation block.J Biol Chem. 2012 Jul 27;287(31):26187-99. doi: 10.1074/jbc.M112.373209. Epub 2012 May 23. J Biol Chem. 2012. PMID: 22621931 Free PMC article.
-
Impact of the epoxide hydrolase EphD on the metabolism of mycolic acids in mycobacteria.J Biol Chem. 2018 Apr 6;293(14):5172-5184. doi: 10.1074/jbc.RA117.000246. Epub 2018 Feb 22. J Biol Chem. 2018. PMID: 29472294 Free PMC article.
References
-
- Brennan P. J., Nikaido H. (1995) Annu. Rev. Biochem. 64, 29–63 - PubMed
-
- Kremer L., Baulard A. R., Besra G. S. (2000) in Molecular Genetics of Mycobacteria (Hatfull G. F., Jacobs W. R., Jr., eds) pp. 173–190, American Society for Microbiology, Washington, D. C.
-
- Daffé M., Draper P. (1998) Adv. Microb. Physiol. 39, 131–203 - PubMed
-
- Glickman M. S., Cox J. S., Jacobs W. R., Jr. (2000) Mol. Cell 5, 717–727 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

