Electrical and chemical synapses between relay neurons in developing thalamus
- PMID: 20457735
- PMCID: PMC2915516
- DOI: 10.1113/jphysiol.2010.187096
Electrical and chemical synapses between relay neurons in developing thalamus
Abstract
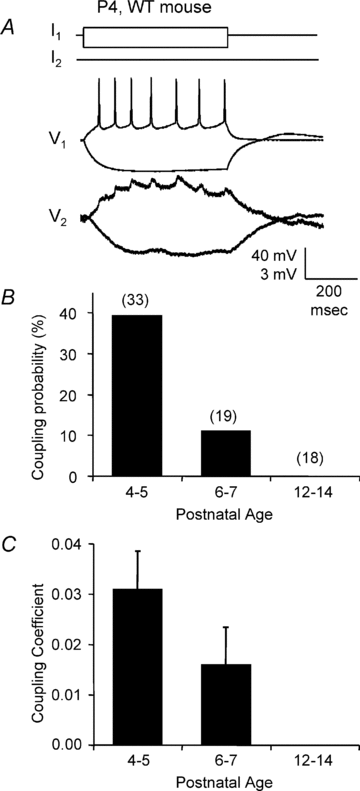
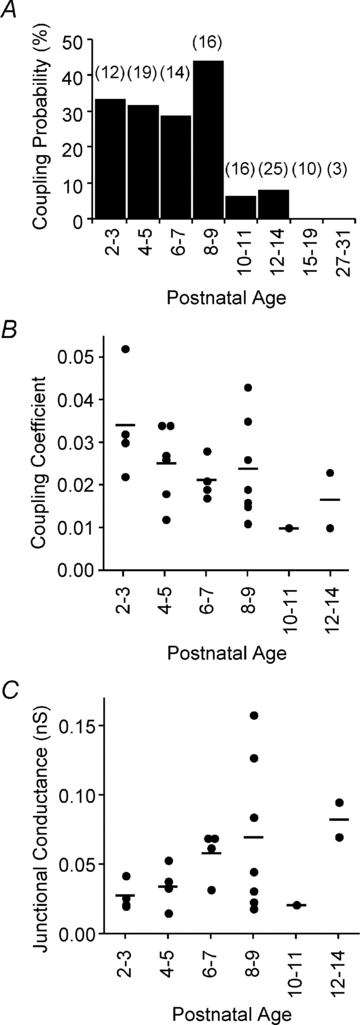
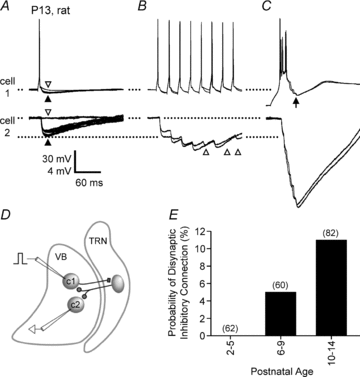
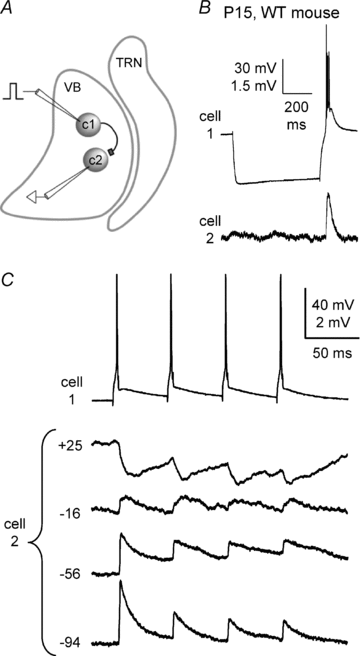
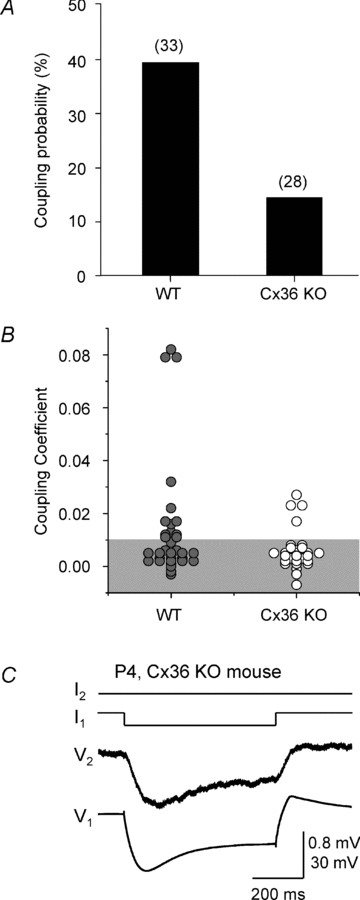
Gap junction-mediated electrical synapses interconnect diverse types of neurons in the mammalian brain, and they may play important roles in the synchronization and development of neural circuits. Thalamic relay neurons are the major source of input to neocortex. Electrical synapses have not been directly observed between relay neurons in either developing or adult animals. We tested for electrical synapses by recording from pairs of relay neurons in acute slices of developing ventrobasal nucleus (VBN) of the thalamus from rats and mice. Electrical synapses were common between VBN relay neurons during the first postnatal week, and then declined sharply during the second week. Electrical coupling was reduced among cells of connexin36 (Cx36) knockout mice; however, some neuron pairs remained coupled. This implies that electrical synapses between the majority of coupled VBN neurons require Cx36 but that other gap junction proteins also contribute. The anatomical distribution of a beta-galactosidase reporter indicated that Cx36 was expressed in some VBN neurons during the first postnatal week and sharply declined over the second week, consistent with our physiological results. VBN relay neurons also communicated via chemical synapses. Rare pairs of relay neurons excited one another monosynaptically. Much more commonly, spikes in one relay neuron evoked disynaptic inhibition (via the thalamic reticular nucleus) in the same or a neighbouring relay neuron. Disynaptic inhibition between VBN cells emerged as electrical coupling was decreasing, during the second postnatal week. Our results demonstrate that thalamic relay neurons communicate primarily via electrical synapses during early postnatal development, and then lose their electrical coupling as a chemical synapse-mediated inhibitory circuit matures.
Figures


References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signalling. Nat Neurosci. 2005;8:1720–1726. - PubMed
-
- Belluardo N, Mudo G, Trovato-Salinaro A, Le Gurun S, Charollais A, Serre-Beinier V, Amato G, Haefliger JA, Meda P, Condorelli DF. Expression of connexin36 in the adult and developing rat brain. Brain Res. 2000;865:121–138. - PubMed
-
- Bennett MV. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

