The archaeal transamidosome for RNA-dependent glutamine biosynthesis
- PMID: 20457752
- PMCID: PMC2943598
- DOI: 10.1093/nar/gkq336
The archaeal transamidosome for RNA-dependent glutamine biosynthesis
Abstract
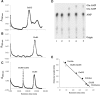
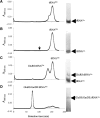
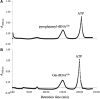
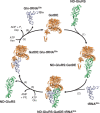
Archaea make glutaminyl-tRNA (Gln-tRNA(Gln)) in a two-step process; a non-discriminating glutamyl-tRNA synthetase (ND-GluRS) forms Glu-tRNA(Gln), while the heterodimeric amidotransferase GatDE converts this mischarged tRNA to Gln-tRNA(Gln). Many prokaryotes synthesize asparaginyl-tRNA (Asn-tRNA(Asn)) in a similar manner using a non-discriminating aspartyl-tRNA synthetase (ND-AspRS) and the heterotrimeric amidotransferase GatCAB. The transamidosome, a complex of tRNA synthetase, amidotransferase and tRNA, was first described for the latter system in Thermus thermophilus [Bailly, M., Blaise, M., Lorber, B., Becker, H.D. and Kern, D. (2007) The transamidosome: a dynamic ribonucleoprotein particle dedicated to prokaryotic tRNA-dependent asparagine biosynthesis. Mol. Cell, 28, 228-239.]. Here, we show a similar complex for Gln-tRNA(Gln) formation in Methanothermobacter thermautotrophicus that allows the mischarged Glu-tRNA(Gln) made by the tRNA synthetase to be channeled to the amidotransferase. The association of archaeal ND-GluRS with GatDE (K(D) = 100 ± 22 nM) sequesters the tRNA synthetase for Gln-tRNA(Gln) formation, with GatDE reducing the affinity of ND-GluRS for tRNA(Glu) by at least 13-fold. Unlike the T. thermophilus transamidosome, the archaeal complex does not require tRNA for its formation, is not stable through product (Gln-tRNA(Gln)) formation, and has no major effect on the kinetics of tRNA(Gln) glutamylation nor transamidation. The differences between the two transamidosomes may be a consequence of the fact that ND-GluRS is a class I aminoacyl-tRNA synthetase, while ND-AspRS belongs to the class II family.
Figures


References
-
- Ibba M, Söll D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000;69:617–650. - PubMed
-
- Becker HD, Reinbolt J, Kreutzer R, Giegé R, Kern D. Existence of two distinct aspartyl-tRNA synthetases in Thermus thermophilus. Structural and biochemical properties of the two enzymes. Biochemistry. 1997;36:8785–8797. - PubMed

