Complement drives Th17 cell differentiation and triggers autoimmune arthritis
- PMID: 20457757
- PMCID: PMC2882841
- DOI: 10.1084/jem.20092301
Complement drives Th17 cell differentiation and triggers autoimmune arthritis
Abstract
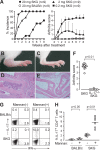
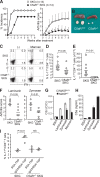
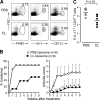
Activation of serum complement triggers Th17 cell-dependent spontaneous autoimmune disease in an animal model. In genetically autoimmune-prone SKG mice, administration of mannan or beta-glucan, both of which activate serum complement, evoked Th17 cell-mediated chronic autoimmune arthritis. C5a, a chief component of complement activation produced via all three complement pathways (i.e., lectin, classical, and alternative), stimulated tissue-resident macrophages, but not dendritic cells, to produce inflammatory cytokines including IL-6, in synergy with Toll-like receptor signaling or, notably, granulocyte/macrophage colony-stimulating factor (GM-CSF). GM-CSF secreted by activated T cells indeed enhanced in vitro IL-6 production by C5a-stimulated macrophages. In vivo, C5a receptor (C5aR) deficiency in SKG mice inhibited the differentiation/expansion of Th17 cells after mannan or beta-glucan treatment, and consequently suppressed the development of arthritis. Transfer of SKG T cells induced Th17 cell differentiation/expansion and produced arthritis in C5aR-sufficient recombination activating gene (RAG)-/- mice but not in C5aR-deficient RAG-/- recipients. In vivo macrophage depletion also inhibited disease development in SKG mice. Collectively, the data suggest that complement activation by exogenous or endogenous stimulation can initiate Th17 cell differentiation and expansion in certain autoimmune diseases and presumably in microbial infections. Blockade of C5aR may thus be beneficial for controlling Th17-mediated inflammation and autoimmune disease.
Figures


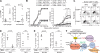
References
-
- Garlatti V., Belloy N., Martin L., Lacroix M., Matsushita M., Endo Y., Fujita T., Fontecilla-Camps J.C., Arlaud G.J., Thielens N.M., Gaboriaud C. 2007. Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J. 26:623–633 10.1038/sj.emboj.7601500 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

