Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin
- PMID: 20457762
- PMCID: PMC2872904
- DOI: 10.1083/jcb.200910119
Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin
Abstract
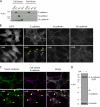
Botulinum neurotoxin is produced by Clostridium botulinum and forms large protein complexes through associations with nontoxic components. We recently found that hemagglutinin (HA), one of the nontoxic components, disrupts the intercellular epithelial barrier; however, the mechanism underlying this phenomenon is not known. In this study, we identified epithelial cadherin (E-cadherin) as a target molecule for HA. HA directly binds E-cadherin and disrupts E-cadherin-mediated cell to cell adhesion. Although HA binds human, bovine, and mouse E-cadherin, it does not bind rat or chicken E-cadherin homologues. HA does not interact with other members of the classical cadherin family such as neural and vascular endothelial cadherin. Expression of rat E-cadherin but not mouse rescues Madin-Darby canine kidney cells from HA-induced tight junction (TJ) disruptions. These data demonstrate that botulinum HA directly binds E-cadherin and disrupts E-cadherin-mediated cell to cell adhesion in a species-specific manner and that the HA-E-cadherin interaction is essential for the disruption of TJ function.
Figures

Similar articles
-
Molecular engineering of a minimal E-cadherin inhibitor protein derived from Clostridium botulinum hemagglutinin.J Biol Chem. 2023 Mar;299(3):102944. doi: 10.1016/j.jbc.2023.102944. Epub 2023 Jan 25. J Biol Chem. 2023. PMID: 36707052 Free PMC article.
-
Clostridium botulinum type C hemagglutinin affects the morphology and viability of cultured mammalian cells via binding to the ganglioside GM3.FEBS J. 2015 Sep;282(17):3334-47. doi: 10.1111/febs.13346. Epub 2015 Jul 14. FEBS J. 2015. PMID: 26077172
-
Functional Analysis of Botulinum Hemagglutinin (HA).Methods Mol Biol. 2020;2132:191-200. doi: 10.1007/978-1-0716-0430-4_20. Methods Mol Biol. 2020. PMID: 32306328
-
Botulinum Hemagglutinin: Critical Protein for Adhesion and Absorption of Neurotoxin Complex in Host Intestine.Methods Mol Biol. 2020;2132:183-190. doi: 10.1007/978-1-0716-0430-4_19. Methods Mol Biol. 2020. PMID: 32306327 Review.
-
[Mechanism of intestinal absorption of botulinum neurotoxin complex].Nihon Saikingaku Zasshi. 2019;74(3):167-175. doi: 10.3412/jsb.74.167. Nihon Saikingaku Zasshi. 2019. PMID: 31787706 Review. Japanese.
Cited by
-
Assembly and function of the botulinum neurotoxin progenitor complex.Curr Top Microbiol Immunol. 2013;364:21-44. doi: 10.1007/978-3-642-33570-9_2. Curr Top Microbiol Immunol. 2013. PMID: 23239347 Free PMC article. Review.
-
Aeromonas sobria serine protease decreases epithelial barrier function in T84 cells and accelerates bacterial translocation across the T84 monolayer in vitro.PLoS One. 2019 Aug 16;14(8):e0221344. doi: 10.1371/journal.pone.0221344. eCollection 2019. PLoS One. 2019. PMID: 31419250 Free PMC article.
-
Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity.PLoS Pathog. 2013;9(10):e1003690. doi: 10.1371/journal.ppat.1003690. Epub 2013 Oct 10. PLoS Pathog. 2013. PMID: 24130488 Free PMC article.
-
Crystallization and preliminary crystallographic studies of the HA3 subcomponent of the type B botulinum neurotoxin complex.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Oct 1;67(Pt 10):1244-6. doi: 10.1107/S1744309111027412. Epub 2011 Sep 29. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22102038 Free PMC article.
-
Characterization of Hemagglutinin Negative Botulinum Progenitor Toxins.Toxins (Basel). 2017 Jun 15;9(6):193. doi: 10.3390/toxins9060193. Toxins (Basel). 2017. PMID: 28617306 Free PMC article.
References
-
- Behrens J., Birchmeier W., Goodman S.L., Imhof B.A. 1985. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J. Cell Biol. 101:1307–1315 10.1083/jcb.101.4.1307 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

