Generation of Trypanosoma cruzi-specific CD8+ T-cell immunity is unaffected by the absence of type I interferon signaling
- PMID: 20457790
- PMCID: PMC2897408
- DOI: 10.1128/IAI.00275-10
Generation of Trypanosoma cruzi-specific CD8+ T-cell immunity is unaffected by the absence of type I interferon signaling
Abstract
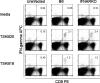
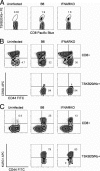
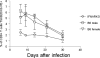
Trypanosoma cruzi is a protozoan parasite that causes human Chagas' disease, a leading source of congestive heart failure in Central and South America. CD8+ T cells are critical for control of T. cruzi infection, and CD8+ T cells recognizing the immunodominant trans-sialidase gene-encoded peptide TSKB20 (ANYKFTLV) account for approximately 30% of the total CD8+ T-cell population at the peak of infection in C57BL/6 mice. Type I interferons (IFN-I) are pleiotropic cytokines that play a critical role in both innate and adaptive immunity against a variety of infections, but their induction and their role in infection are dictated by the infectious agent. Because type I IFNs and IFN-responsive genes are evident early after T. cruzi infection of host cells, we examined the influence of IFN-I on the development of CD8+ T-cell responses during this infection. Mice lacking the receptor for IFN-I (IFNARKO) and their wild-type counterparts both developed chronic infections and generated similar frequencies of immunodominant TSKB20- and subdominant TSKB18-specific CD8+ T cells following T. cruzi infection. In contrast, peak TSKB20-specific CD8+ T-cell responses generated during infection with vaccinia virus engineered to express TSKB20 were approximately 2.5-fold lower in IFNARKO mice than B6 mice, although after viral clearance, the frequencies of TSKB20-specific CD8+ T cells stabilized at similar levels. Together, these data suggest that IFN-I induction and biology are dependent upon the microbial context and emphasize the need to investigate various infection models for a full understanding of CD8+ T-cell development.
Figures


Similar articles
-
Long-Term Immunity to Trypanosoma cruzi in the Absence of Immunodominant trans-Sialidase-Specific CD8+ T Cells.Infect Immun. 2016 Aug 19;84(9):2627-38. doi: 10.1128/IAI.00241-16. Print 2016 Sep. Infect Immun. 2016. PMID: 27354447 Free PMC article.
-
CD8+ T cells specific for immunodominant trans-sialidase epitopes contribute to control of Trypanosoma cruzi infection but are not required for resistance.J Immunol. 2010 Jul 1;185(1):560-8. doi: 10.4049/jimmunol.1000432. Epub 2010 Jun 7. J Immunol. 2010. PMID: 20530265 Free PMC article.
-
Limited role for CD4+ T-cell help in the initial priming of Trypanosoma cruzi-specific CD8+ T cells.Infect Immun. 2007 Jan;75(1):231-5. doi: 10.1128/IAI.01245-06. Epub 2006 Oct 16. Infect Immun. 2007. PMID: 17043105 Free PMC article.
-
Generation, specificity, and function of CD8+ T cells in Trypanosoma cruzi infection.Immunol Rev. 2004 Oct;201:304-17. doi: 10.1111/j.0105-2896.2004.00183.x. Immunol Rev. 2004. PMID: 15361249 Review.
-
CD8(+) T cell-mediated immunity during Trypanosoma cruzi infection: a path for vaccine development?Mediators Inflamm. 2014;2014:243786. doi: 10.1155/2014/243786. Epub 2014 Jul 1. Mediators Inflamm. 2014. PMID: 25104879 Free PMC article. Review.
Cited by
-
Understanding CD8+ T Cell Immunity to Trypanosoma cruzi and How to Improve It.Trends Parasitol. 2019 Nov;35(11):899-917. doi: 10.1016/j.pt.2019.08.006. Epub 2019 Oct 10. Trends Parasitol. 2019. PMID: 31607632 Free PMC article. Review.
-
Pathogenesis of chagas' disease: parasite persistence and autoimmunity.Clin Microbiol Rev. 2011 Jul;24(3):592-630. doi: 10.1128/CMR.00063-10. Clin Microbiol Rev. 2011. PMID: 21734249 Free PMC article. Review.
-
Trypanosoma cruzi Causes Paralyzing Systemic Necrotizing Vasculitis Driven by Pathogen-Specific Type I Immunity in Mice.Infect Immun. 2016 Mar 24;84(4):1123-1136. doi: 10.1128/IAI.01497-15. Print 2016 Apr. Infect Immun. 2016. PMID: 26857570 Free PMC article.
-
Invasion and intracellular survival by protozoan parasites.Immunol Rev. 2011 Mar;240(1):72-91. doi: 10.1111/j.1600-065X.2010.00990.x. Immunol Rev. 2011. PMID: 21349087 Free PMC article. Review.
-
The importance of persistence and dormancy in Trypanosoma cruzi infection and Chagas disease.Curr Opin Microbiol. 2025 Aug;86:102615. doi: 10.1016/j.mib.2025.102615. Epub 2025 Jun 6. Curr Opin Microbiol. 2025. PMID: 40482566 Review.
References
-
- Aichele, P., H. Unsoeld, M. Koschella, O. Schweier, U. Kalinke, and S. Vucikuja. 2006. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J. Immunol. 176:4525-4529. - PubMed
-
- Chessler, A. D., M. Unnikrishnan, A. K. Bei, J. P. Daily, and B. A. Burleigh. 2009. Trypanosoma cruzi triggers an early type I IFN response in vivo at the site of intradermal infection. J. Immunol. 182:2288-2296. - PubMed
-
- El-Sayed, N. M., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C. Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D. Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409-415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

