Cholangiocyte myosin IIB is required for localized aggregation of sodium glucose cotransporter 1 to sites of Cryptosporidium parvum cellular invasion and facilitates parasite internalization
- PMID: 20457792
- PMCID: PMC2897363
- DOI: 10.1128/IAI.00077-10
Cholangiocyte myosin IIB is required for localized aggregation of sodium glucose cotransporter 1 to sites of Cryptosporidium parvum cellular invasion and facilitates parasite internalization
Abstract
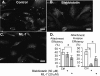
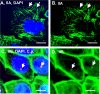
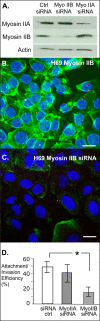
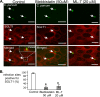
Internalization of the obligate intracellular apicomplexan parasite, Cryptosporidium parvum, results in the formation of a unique intramembranous yet extracytoplasmic niche on the apical surfaces of host epithelial cells, a process that depends on host cell membrane extension. We previously demonstrated that efficient C. parvum invasion of biliary epithelial cells (cholangiocytes) requires host cell actin polymerization and localized membrane translocation/insertion of Na(+)/glucose cotransporter 1 (SGLT1) and of aquaporin 1 (Aqp1), a water channel, at the attachment site. The resultant localized water influx facilitates parasite cellular invasion by promoting host-cell membrane protrusion. However, the molecular mechanisms by which C. parvum induces membrane translocation/insertion of SGLT1/Aqp1 are obscure. We report here that cultured human cholangiocytes express several nonmuscle myosins, including myosins IIA and IIB. Moreover, C. parvum infection of cultured cholangiocytes results in the localized selective aggregation of myosin IIB but not myosin IIA at the region of parasite attachment, as assessed by dual-label immunofluorescence confocal microscopy. Concordantly, treatment of cells with the myosin light chain kinase inhibitor ML-7 or the myosin II-specific inhibitor blebbistatin or selective RNA-mediated repression of myosin IIB significantly inhibits (P < 0.05) C. parvum cellular invasion (by 60 to 80%). Furthermore ML-7 and blebbistatin significantly decrease (P < 0.02) C. parvum-induced accumulation of SGLT1 at infection sites (by approximately 80%). Thus, localized actomyosin-dependent membrane translocation of transporters/channels initiated by C. parvum is essential for membrane extension and parasite internalization, a phenomenon that may also be relevant to the mechanisms of cell membrane protrusion in general.
Figures



References
-
- Aji, T., T. Flanigan, R. Marshall, C. Kaetzel, and M. Aikawa. 1991. Ultrastructural study of asexual development of Cryptosporidium parvum in a human intestinal cell line. J. Protozool. 38:82S-84S. - PubMed
-
- Bao, J., S. S. Jana, and R. S. Adelstein. 2005. Vertebrate nonmuscle myosin II isoforms rescue small interfering RNA-induced defects in COS-7 cell cytokinesis. J. Biol. Chem. 280:19594-19599. - PubMed
-
- Bao, J., X. Ma, C. Liu, and R. S. Adelstein. 2007. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J. Biol. Chem. 282:22102-22111. - PubMed
-
- Bonnin, A., A. Lapillonne, T. Petrella, J. Lopez, C. Chaponnier, G. Gabbiani, S. Robine, and J. F. Dubremetz. 1999. Immunodetection of the microvillous cytoskeleton molecules villin and ezrin in the parasitophorous vacuole wall of Cryptosporidium parvum (Protozoa: Apicomplexa). Eur. J. Cell Biol. 78:794-801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

