Imaging myocardial metabolic remodeling
- PMID: 20457796
- PMCID: PMC3427939
- DOI: 10.2967/jnumed.109.068197
Imaging myocardial metabolic remodeling
Abstract
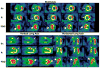
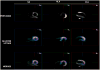
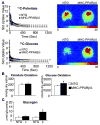
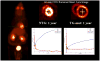
Myocardial metabolic remodeling is the process in which the heart loses its ability to utilize different substrates, becoming dependent primarily on the metabolism of a single substrate such as glucose or fatty acids for energy production. Myocardial metabolic remodeling is central to the pathogenesis of a variety of cardiac disease processes such as left ventricular hypertrophy, myocardial ischemia, and diabetic cardiomyopathy. As a consequence, there is a growing demand for accurate noninvasive imaging approaches of various aspects of myocardial substrate metabolism that can be performed in both humans and small-animal models of disease, facilitating the crosstalk between the bedside and the bench and leading to improved patient management paradigms. SPECT, PET, and MR spectroscopy are the most commonly used imaging techniques. Discussed in this review are the strengths and weaknesses of these various imaging methods and how they are furthering our understanding of the role of myocardial remodeling in cardiovascular disease. In addition, the role of ultrasound to detect the inflammatory response to myocardial ischemia will be discussed.
Figures


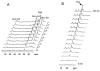
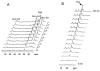



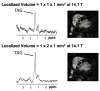



References
-
- He ZX, Shi RF, Wu YJ, Tian YQ, Liu XJ, Wang SW, Shen R, Qin XW, Gao RL, Narula J, Jain D. Direct imaging of exercise-induced myocardial ischemia with fluorine-18-labeled deoxyglucose and Tc-99m-sestamibi in coronary artery disease. Circulation. 2003;108:1208–1213. - PubMed
-
- Bergmann SR, Weinheimer CJ, Markham J, Herrero P. Quantitation of myocardial fatty acid metabolism using PET. J Nucl Med. 1996;37:1723–1730. - PubMed
-
- Herrero P, Dence CS, Coggan AR, Kisrieva-Ware Z, Eisenbeis P, Gropler RJ. L-3-11C-lactate as a PET tracer of myocardial lactate metabolism: a feasibility study. J Nucl Med. 2007;48:2046–2055. - PubMed
-
- Herrero P, Kisrieva-Ware Z, Dence CS, Patterson B, Coggan AR, Han DH, Ishii Y, Eisenbeis P, Gropler RJ. PET measurements of myocardial glucose metabolism with 1-11C-glucose and kinetic modeling. J Nucl Med. 2007;48:955–964. - PubMed
-
- Hudsmith LE, Neubauer S. Magnetic resonance spectroscopy in myocardial disease. JACC Cardiovasc Imaging. 2009;2:87–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL056178/HL/NHLBI NIH HHS/United States
- R01HL62702/HL/NHLBI NIH HHS/United States
- R01HL077434/HL/NHLBI NIH HHS/United States
- R01 HL062702/HL/NHLBI NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- R37HL49244/HL/NHLBI NIH HHS/United States
- P01 HL013851/HL/NHLBI NIH HHS/United States
- HL69100/HL/NHLBI NIH HHS/United States
- R01 EB012284/EB/NIBIB NIH HHS/United States
- R37 HL049244/HL/NHLBI NIH HHS/United States
- P01-HL13851/HL/NHLBI NIH HHS/United States
- R01 HL069100/HL/NHLBI NIH HHS/United States
- R01HL56178/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
