Pref-1 interacts with fibronectin to inhibit adipocyte differentiation
- PMID: 20457810
- PMCID: PMC2897553
- DOI: 10.1128/MCB.00057-10
Pref-1 interacts with fibronectin to inhibit adipocyte differentiation
Abstract
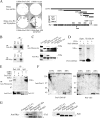
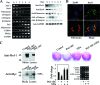
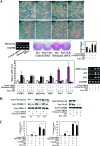
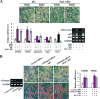
Pref-1/Dlk1 is made as an epidermal growth factor (EGF) repeat-containing transmembrane protein but is cleaved by tumor necrosis factor alpha converting enzyme (TACE) to generate a biologically active soluble form. Soluble Pref-1 inhibits adipocyte differentiation through the activation of extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) and the subsequent upregulation of Sox9 expression. However, others have implicated Notch in Pref-1 signaling and function. Here, we show that Pref-1 does not interact with, or require, Notch for its function. Instead, we show a direct interaction of Pref-1 and fibronectin via the Pref-1 juxtamembrane domain and fibronectin C-terminal domain. We also show that fibronectin is required for the Pref-1-mediated inhibition of adipocyte differentiation, the activation of ERK/MAPK, and the upregulation of Sox9. Furthermore, disrupting fibronectin binding to integrin by the addition of RGD peptides or by the knockdown of alpha 5 integrin prevents the Pref-1 inhibition of adipocyte differentiation. Pref-1 activates the integrin downstream signaling molecules, FAK and Rac, and ERK activation by Pref-1 is blunted by the knockdown of Rac or by the forced expression of dominant-negative Rac. We conclude that, by interacting with fibronectin, Pref-1 activates integrin downstream signaling to activate MEK/ERK and to inhibit adipocyte differentiation.
Figures

Similar articles
-
Pref-1 (preadipocyte factor 1) activates the MEK/extracellular signal-regulated kinase pathway to inhibit adipocyte differentiation.Mol Cell Biol. 2007 Mar;27(6):2294-308. doi: 10.1128/MCB.02207-06. Epub 2007 Jan 8. Mol Cell Biol. 2007. PMID: 17210639 Free PMC article.
-
Insulin-like growth factor-1/insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation.J Biol Chem. 2003 Jun 6;278(23):20906-14. doi: 10.1074/jbc.M300022200. Epub 2003 Mar 21. J Biol Chem. 2003. PMID: 12651852
-
Minireview: Pref-1: role in adipogenesis and mesenchymal cell fate.Mol Endocrinol. 2009 Nov;23(11):1717-25. doi: 10.1210/me.2009-0160. Epub 2009 Jun 18. Mol Endocrinol. 2009. PMID: 19541743 Free PMC article. Review.
-
Pref-1 regulates mesenchymal cell commitment and differentiation through Sox9.Cell Metab. 2009 Mar;9(3):287-302. doi: 10.1016/j.cmet.2009.01.013. Cell Metab. 2009. PMID: 19254573 Free PMC article.
-
Function of pref-1 as an inhibitor of adipocyte differentiation.Int J Obes Relat Metab Disord. 2000 Nov;24 Suppl 4:S15-9. doi: 10.1038/sj.ijo.0801494. Int J Obes Relat Metab Disord. 2000. PMID: 11126233 Review.
Cited by
-
Pref-1, a gatekeeper of adipogenesis.Front Endocrinol (Lausanne). 2013 Jul 3;4:79. doi: 10.3389/fendo.2013.00079. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23840193 Free PMC article.
-
Fibronectin and stem cell differentiation - lessons from chondrogenesis.J Cell Sci. 2012 Aug 15;125(Pt 16):3703-12. doi: 10.1242/jcs.095786. Epub 2012 Sep 12. J Cell Sci. 2012. PMID: 22976308 Free PMC article. Review.
-
The imprinted gene Delta like non-canonical notch ligand 1 (Dlk1) associates with obesity and triggers insulin resistance through inhibition of skeletal muscle glucose uptake.EBioMedicine. 2019 Aug;46:368-380. doi: 10.1016/j.ebiom.2019.07.070. Epub 2019 Aug 3. EBioMedicine. 2019. PMID: 31383551 Free PMC article.
-
Prenatal notch1 receptor blockade by protein delta homolog 1 (DLK1) modulates adipocyte size in vivo.Int J Obes (Lond). 2016 Apr;40(4):698-705. doi: 10.1038/ijo.2015.227. Epub 2015 Aug 26. Int J Obes (Lond). 2016. PMID: 26499442
-
Mechanotransduction through adhesion molecules: Emerging roles in regulating the stem cell niche.Front Cell Dev Biol. 2022 Sep 12;10:966662. doi: 10.3389/fcell.2022.966662. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172276 Free PMC article. Review.
References
-
- Antras, J., F. Hilliou, G. Redziniak, and J. Pairault. 1989. Decreased biosynthesis of actin and cellular fibronectin during adipose conversion of 3T3-F442A cells. Reorganization of the cytoarchitecture and extracellular matrix fibronectin. Biol. Cell 66:247-254. - PubMed
-
- Baladrón, V., M. J. Ruiz-Hidalgo, M. L. Nueda, M. J. Diaz-Guerra, J. J. Garcia-Ramirez, E. Bonvini, E. Gubina, and J. Laborda. 2005. dlk acts as a negative regulator of Notch1 activation through interactions with specific EGF-like repeats. Exp. Cell Res. 303:343-359. - PubMed
-
- Fukai, F., T. Iso, K. Sekiguchi, N. Miyatake, A. Tsugita, and T. Katayama. 1993. An amino-terminal fibronectin fragment stimulates the differentiation of ST-13 preadipocytes. Biochemistry 32:5746-5751. - PubMed
-
- Garcés, C., M. J. Ruiz-Hidalgo, J. Font de Mora, C. Park, L. Miele, J. Goldstein, E. Bonvini, A. Porras, and J. Laborda. 1997. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J. Biol. Chem. 272:29729-29734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
