The POU transcription factor Drifter/Ventral veinless regulates expression of Drosophila immune defense genes
- PMID: 20457811
- PMCID: PMC2897550
- DOI: 10.1128/MCB.00223-10
The POU transcription factor Drifter/Ventral veinless regulates expression of Drosophila immune defense genes
Abstract
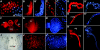
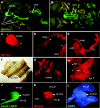
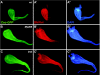
Innate immunity operates as a first line of defense in multicellular organisms against infections caused by different classes of microorganisms. Antimicrobial peptides (AMPs) are synthesized constitutively in barrier epithelia to protect against microbial attack and are also upregulated in response to infection. Here, we implicate Drifter/Ventral veinless (Dfr/Vvl), a class III POU domain transcription factor, in tissue-specific regulation of the innate immune defense of Drosophila. We show that Dfr/Vvl is highly expressed in a range of immunocompetent tissues, including the male ejaculatory duct, where its presence overlaps with and drives the expression of cecropin, a potent broad-spectrum AMP. Dfr/Vvl overexpression activates transcription of several AMP genes in uninfected flies in a Toll pathway- and Imd pathway-independent manner. Dfr/Vvl activates a CecA1 reporter gene both in vitro and in vivo by binding to an upstream enhancer specific for the male ejaculatory duct. Further, Dfr/Vvl and the homeodomain protein Caudal (Cad) activate transcription synergistically via this enhancer. We propose that the POU protein Dfr/Vvl acts together with other regulators in a combinatorial manner to control constitutive AMP gene expression in a gene-, tissue-, and sex-specific manner, thus promoting a first-line defense against infection in tissues that are readily exposed to pathogens.
Figures


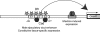
Similar articles
-
Isolation of regulators of Drosophila immune defense genes by a double interaction screen in yeast.Insect Biochem Mol Biol. 2007 Mar;37(3):202-12. doi: 10.1016/j.ibmb.2006.10.008. Epub 2006 Nov 7. Insect Biochem Mol Biol. 2007. PMID: 17296495
-
Stop codon readthrough alters the activity of a POU/Oct transcription factor during Drosophila development.BMC Biol. 2021 Sep 3;19(1):185. doi: 10.1186/s12915-021-01106-0. BMC Biol. 2021. PMID: 34479564 Free PMC article.
-
ventral veinless, a POU domain transcription factor, regulates different transduction pathways required for tracheal branching in Drosophila.Development. 1997 Sep;124(17):3273-81. doi: 10.1242/dev.124.17.3273. Development. 1997. PMID: 9310322
-
Regulation of immune and tissue homeostasis by Drosophila POU factors.Insect Biochem Mol Biol. 2019 Jun;109:24-30. doi: 10.1016/j.ibmb.2019.04.003. Epub 2019 Apr 5. Insect Biochem Mol Biol. 2019. PMID: 30954681 Review.
-
A multilayered defense against infection: combinatorial control of insect immune genes.Trends Genet. 2007 Jul;23(7):342-9. doi: 10.1016/j.tig.2007.05.003. Epub 2007 May 29. Trends Genet. 2007. PMID: 17532525 Review.
Cited by
-
Crossveinless is a direct transcriptional target of Trachealess and Tango in Drosophila tracheal precursor cells.PLoS One. 2019 Jun 3;14(6):e0217906. doi: 10.1371/journal.pone.0217906. eCollection 2019. PLoS One. 2019. PMID: 31158257 Free PMC article.
-
Transcriptional control of steroid biosynthesis genes in the Drosophila prothoracic gland by ventral veins lacking and knirps.PLoS Genet. 2014 Jun 19;10(6):e1004343. doi: 10.1371/journal.pgen.1004343. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24945799 Free PMC article.
-
The Drosophila IMD pathway in the activation of the humoral immune response.Dev Comp Immunol. 2014 Jan;42(1):25-35. doi: 10.1016/j.dci.2013.05.014. Epub 2013 May 27. Dev Comp Immunol. 2014. PMID: 23721820 Free PMC article. Review.
-
Immune response in the barrier epithelia: lessons from the fruit fly Drosophila melanogaster.J Innate Immun. 2012;4(3):273-83. doi: 10.1159/000332947. Epub 2012 Jan 10. J Innate Immun. 2012. PMID: 22237424 Free PMC article. Review.
-
The POU Transcription Factor POU-M2 Regulates Vitellogenin Receptor Gene Expression in the Silkworm, Bombyx mori.Genes (Basel). 2020 Apr 6;11(4):394. doi: 10.3390/genes11040394. Genes (Basel). 2020. PMID: 32268540 Free PMC article.
References
-
- Agerberth, B., and G. H. Gudmundsson. 2006. Host antimicrobial defence peptides in human disease. Curr. Top. Microbiol. Immunol. 306:67-90. - PubMed
-
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
-
- Andersen, B., and M. G. Rosenfeld. 2001. POU domain factors in the neuroendocrine system: lessons from developmental biology provide insights into human disease. Endocr. Rev. 22:2-35. - PubMed
-
- Anderson, M. G., S. J. Certel, K. Certel, T. Lee, D. J. Montell, and W. A. Johnson. 1996. Function of the Drosophila POU domain transcription factor drifter as an upstream regulator of breathless receptor tyrosine kinase expression in developing trachea. Development 122:4169-4178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
