Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions
- PMID: 20457817
- PMCID: PMC2916350
- DOI: 10.1128/AAC.01753-09
Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions
Abstract
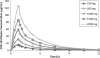
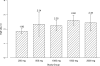
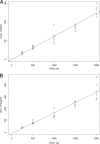
CXA-101 is a novel, broad-spectrum cephalosporin with excellent antipseudomonal activity. A Phase 1 study was performed to determine the safety, tolerability, and pharmacokinetics of CXA-101 after single- and multiple-dose intravenous administration over 1 h to healthy male and female subjects. In part 1 of the study, five cohorts of eight subjects each (six receiving CXA-101 and two receiving a placebo) received single ascending doses of 250, 500, 1,000, 1,500, and 2,000 mg. In part 2, cohorts 1 and 2 received 500 mg and 1,000 mg, respectively, every 8 h, and cohort 3 received 1,500 mg every 12 h; each cohort received dosing for 10 days. Standard safety and tolerability assessments were performed. Blood and urine pharmacokinetic samples were assayed by a validated bioanalytical method and analyzed using standard noncompartmental methodology. All 64 subjects completed dosing; none withdrew from the study. Drug-related systemic adverse events were infrequent and mild. Mild, non-treatment-limiting infusion site events occurred during multiple-dose administration. No clinically significant laboratory or electrocardiographic finding or dose-limiting toxicity was observed. CXA-101 exhibited dose-linear pharmacokinetics; the mean plasma half-life was approximately 2.3 h. More than 90% of the administered dose was eliminated unchanged through renal excretion. In summary, CXA-101 administered as a 1-hour infusion was generally safe and well tolerated in single doses up to 2,000 mg and in multiple doses up to 3 g daily over 10 days. The favorable safety and predictable pharmacokinetic profile of CXA-101 support its continuing clinical development for the treatment of serious bacterial infections.
Figures
References
-
- Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, Jr., D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. - PubMed
-
- Brown, N. P., C. M. Pillar, D. C. Draghi, M. K. Torres, C. Thornsberry, D. F. Sahm, and Y. Ge. 2008. Activity profile of CXA-101 against gram-positive and gram-negative pathogens by broth and agar dilution, abstr. F1-354. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. Washington, DC, 25 to 28 October 2008.
-
- Brown, N. P., C. M. Pillar, D. F. Sahm, and Y. Ge. 2009. Activity profile of CXA-101 and CXA-101/tazobactam against target gram-positive and gram-negative pathogens, abstr. F1-1986. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother., San Francisco, CA, 12 to 15 September 2009.
-
- European Medicines Agency. 2009. The bacterial challenge: time to react. A call to narrow the gap between multidrug-resistant bacteria in the EU and the development of new antibacterial agents. ECDC/EMEA joint technical report. http://www.emea.europa.eu/pdfs/human/antimicrobial_resistance/EMEA-57617....
-
- Falagas, M. E., and I. A. Bliziotis. 2007. Pandrug-resistant gram-negative bacteria: the dawn of the post-antibiotic era? Int. J. Antimicrob. Agents 29:630-636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

