Visual error signals from the pretectal nucleus of the optic tract guide motor learning for smooth pursuit
- PMID: 20457849
- PMCID: PMC2867559
- DOI: 10.1152/jn.01024.2009
Visual error signals from the pretectal nucleus of the optic tract guide motor learning for smooth pursuit
Abstract
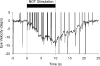
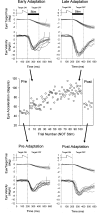
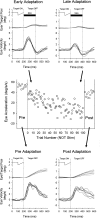
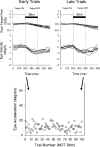
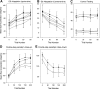
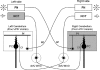
Smooth pursuit (SP) eye movements are used to maintain the image of a moving object on or near the fovea. Visual motion signals aid in driving SP and are necessary for its adaptation. The sources of visual error signals that support SP adaptation are incompletely understood but could involve neurons in cortical and brain stem areas with direction selective visual motion responses. Here we focus on the pretectal nucleus of the optic tract (NOT), which encodes retinal error information during SP. The aim of this study was to characterize the role of the NOT in SP adaptation. SP adaptation is typically produced using a double step of velocity ramp (double-step paradigm), where target speed either increases or decreases 100 ms after the beginning of a trial. In our study, we delivered a brief (200 ms) train of microelectrical stimulation (ES) in the left NOT to introduce directional error signals at the point in time where a second target speed would appear in a double-step paradigm. The target was extinguished coincidentally with the onset of the ES train. Initial eye acceleration (1st 100 ms) showed significant increases after 100 trials, which included left NOT stimulation during ongoing pursuit in an ipsiversive (leftward) direction. In contrast, initial eye acceleration showed significant decreases after repeated left NOT stimulation during contraversive (rightward) SP. Control studies performed using the same periodicity of NOT stimulation as in the preceding text but without accompanying SP did not induce changes in eye acceleration. In contrast, ES of the NOT paired with active SP produced gradual changes in eye acceleration similar to that observed in double-step paradigm. Therefore our findings support the suggestion that the NOT is an important source of visual error information for guiding motor learning during horizontal SP.
Figures



Similar articles
-
Predictive responses of periarcuate pursuit neurons to visual target motion.Exp Brain Res. 2002 Jul;145(1):104-20. doi: 10.1007/s00221-002-1088-7. Epub 2002 Apr 24. Exp Brain Res. 2002. PMID: 12070750
-
Temporal properties of visual motion signals for the initiation of smooth pursuit eye movements in monkeys.J Neurophysiol. 1994 Jul;72(1):150-62. doi: 10.1152/jn.1994.72.1.150. J Neurophysiol. 1994. PMID: 7965001
-
Discharge patterns of neurons in the pretectal nucleus of the optic tract (NOT) in the behaving primate.J Neurophysiol. 1990 Jul;64(1):77-90. doi: 10.1152/jn.1990.64.1.77. J Neurophysiol. 1990. PMID: 2388076
-
The neuronal basis of on-line visual control in smooth pursuit eye movements.Vision Res. 2015 May;110(Pt B):257-64. doi: 10.1016/j.visres.2014.06.008. Epub 2014 Jul 1. Vision Res. 2015. PMID: 24995378 Free PMC article. Review.
-
Signal processing and distribution in cortical-brainstem pathways for smooth pursuit eye movements.Ann N Y Acad Sci. 2009 May;1164:147-54. doi: 10.1111/j.1749-6632.2009.03859.x. Ann N Y Acad Sci. 2009. PMID: 19645893 Free PMC article. Review.
Cited by
-
Conjugate adaptation of smooth pursuit during monocular viewing in strabismic monkeys with exotropia.Invest Ophthalmol Vis Sci. 2012 Apr 24;53(4):2038-45. doi: 10.1167/iovs.11-9011. Invest Ophthalmol Vis Sci. 2012. PMID: 22410567 Free PMC article.
-
Temporal dynamics of retinal and extraretinal signals in the FEFsem during smooth pursuit eye movements.J Neurophysiol. 2017 May 1;117(5):1987-2003. doi: 10.1152/jn.00786.2016. Epub 2017 Feb 15. J Neurophysiol. 2017. PMID: 28202571 Free PMC article.
-
Response properties of MST parafoveal neurons during smooth pursuit adaptation.J Neurophysiol. 2016 Jul 1;116(1):210-7. doi: 10.1152/jn.00203.2016. Epub 2016 Apr 20. J Neurophysiol. 2016. PMID: 27098026 Free PMC article.
-
Short-term adaptations of the dynamic disparity vergence and phoria systems.Exp Brain Res. 2011 Jul;212(2):267-78. doi: 10.1007/s00221-011-2727-7. Epub 2011 May 19. Exp Brain Res. 2011. PMID: 21594645 Clinical Trial.
-
FEFsem neuronal response during combined volitional and reflexive pursuit.J Vis. 2017 May 1;17(5):13. doi: 10.1167/17.5.13. J Vis. 2017. PMID: 28538993 Free PMC article.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci 10: 25–61, 1971.
-
- Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol 204: 44–55, 1982. - PubMed
-
- Buttner-Ennever JA, Cohen B, Horn AK, Reisine H. Efferent pathways of the nucleus of the optic tract in monkey and their role in eye movements. J Comp Neurol 373: 90–107, 1996. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

