Co-induction of LTP and LTD and its regulation by protein kinases and phosphatases
- PMID: 20457859
- PMCID: PMC2867558
- DOI: 10.1152/jn.01112.2009
Co-induction of LTP and LTD and its regulation by protein kinases and phosphatases
Abstract
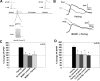
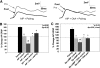
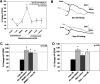
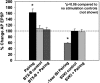
The cellular properties of long-term potentiation (LTP) following pairing of pre- and postsynaptic activity were examined at a known glutamatergic synapse in the leech, specifically between the pressure (P) mechanosensory and anterior pagoda (AP) neurons. Stimulation of the presynaptic P cell (25 Hz) concurrent with a 2 nA depolarization of the postsynaptic AP cell significantly potentiated the P-to-AP excitatory postsynaptic potential (EPSP) in an N-methyl-d-aspartate receptor (NMDAR)-dependent manner based on inhibitory effects of the NMDAR antagonist MK801 and inhibition of the NMDAR glycine binding site by 7-chlorokynurenic acid. LTP was blocked by injection of bis-(o-aminophenoxy)-N,N,N',N'-tetraacetic acid (BAPTA) into the postsynaptic (AP) cell, indicating a requirement for postsynaptic elevation of intracellular Ca(2+). Autocamtide-2-related inhibitory peptide (AIP), a specific inhibitor of Ca(2+)/calmodulin-dependent kinase II (CaMKII), and Rp-cAMP, an inhibitor of protein kinase A (PKA), also blocked pairing-induced potentiation, indicating a requirement for activation of CaMKII and PKA. Interestingly, application of AIP during pairing resulted in significantly depressed synaptic transmission. Co-application of AIP with the protein phosphatase inhibitor okadaic acid restored synaptic transmission to baseline levels, suggesting an interaction between CaMKII and protein phosphatases during induction of activity-dependent synaptic plasticity. When postsynaptic activity preceded presynaptic activity, NMDAR-dependent long-term depression (LTD) was observed that was blocked by okadaic acid. Postsynaptic injection of botulinum toxin blocked P-to-AP potentiation while postsynaptic injection of pep2-SVKI, an inhibitor of AMPA receptor endocytosis, inhibited LTD, supporting the hypothesis that glutamate receptor trafficking contributes to both LTP and LTD at the P-to-AP synapse in the leech.
Figures

Similar articles
-
Forskolin induces NMDA receptor-dependent potentiation at a central synapse in the leech.J Neurophysiol. 2008 May;99(5):2719-24. doi: 10.1152/jn.00010.2008. Epub 2008 Mar 12. J Neurophysiol. 2008. PMID: 18337371
-
CNQX and AMPA inhibit electrical synaptic transmission: a potential interaction between electrical and glutamatergic synapses.Brain Res. 2008 Sep 4;1228:43-57. doi: 10.1016/j.brainres.2008.06.035. Epub 2008 Jun 20. Brain Res. 2008. PMID: 18601913 Free PMC article.
-
Synapse-specific compartmentalization of signaling cascades for LTP induction in CA3 interneurons.Neuroscience. 2015 Apr 2;290:332-45. doi: 10.1016/j.neuroscience.2015.01.024. Epub 2015 Jan 28. Neuroscience. 2015. PMID: 25637803 Free PMC article.
-
Long-term potentiation in cultured hippocampal neurons.Semin Cell Dev Biol. 2011 Jul;22(5):506-13. doi: 10.1016/j.semcdb.2011.07.017. Epub 2011 Jul 22. Semin Cell Dev Biol. 2011. PMID: 21807105 Review.
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
Cited by
-
Interaction between NMDA Receptor- and Endocannabinoid-Mediated Modulation of Nociceptive Synapses.Sci Rep. 2019 Feb 4;9(1):1373. doi: 10.1038/s41598-018-37890-z. Sci Rep. 2019. PMID: 30718662 Free PMC article.
-
Comparative biology of pain: What invertebrates can tell us about how nociception works.J Neurophysiol. 2017 Apr 1;117(4):1461-1473. doi: 10.1152/jn.00600.2016. Epub 2017 Jan 4. J Neurophysiol. 2017. PMID: 28053241 Free PMC article. Review.
-
Neuronal processing of noxious thermal stimuli mediated by dendritic Ca(2+) influx in Drosophila somatosensory neurons.Elife. 2016 Feb 15;5:e12959. doi: 10.7554/eLife.12959. Elife. 2016. PMID: 26880554 Free PMC article.
-
Properties of cannabinoid-dependent long-term depression in the leech.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Nov;196(11):841-51. doi: 10.1007/s00359-010-0566-9. Epub 2010 Aug 28. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20803022
-
Seasonal variation of long-term potentiation at a central synapse in the medicinal leech.J Exp Biol. 2011 Aug 1;214(Pt 15):2534-9. doi: 10.1242/jeb.057224. J Exp Biol. 2011. PMID: 21753047 Free PMC article.
References
-
- Anwyl R. Induction and expression mechanisms of postsynaptic NMDA receptor-independent homosynaptic long-term depression. Prog Neurobiol 78: 17–37, 2006. - PubMed
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3: 1291–1300, 2000. - PubMed
-
- Beck A, Lohr C, Deitmer JW. Calcium transients in subcompartments of the leech Retzius neuron as induced by single action potentials. J Neurobiol 48: 1–18, 2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

