The role of V1 surround suppression in MT motion integration
- PMID: 20457860
- PMCID: PMC2888240
- DOI: 10.1152/jn.00654.2009
The role of V1 surround suppression in MT motion integration
Abstract
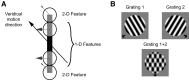
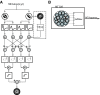
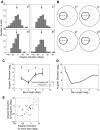
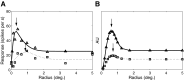
Neurons in the primate extrastriate cortex are highly selective for complex stimulus features such as faces, objects, and motion patterns. One explanation for this selectivity is that neurons in these areas carry out sophisticated computations on the outputs of lower-level areas such as primary visual cortex (V1), where neuronal selectivity is often modeled in terms of linear spatiotemporal filters. However, it has long been known that such simple V1 models are incomplete because they fail to capture important nonlinearities that can substantially alter neuronal selectivity for specific stimulus features. Thus a key step in understanding the function of higher cortical areas is the development of realistic models of their V1 inputs. We have addressed this issue by constructing a computational model of the V1 neurons that provide the strongest input to extrastriate cortical middle temporal (MT) area. We find that a modest elaboration to the standard model of V1 direction selectivity generates model neurons with strong end-stopping, a property that is also found in the V1 layers that provide input to MT. With this computational feature in place, the seemingly complex properties of MT neurons can be simulated by assuming that they perform a simple nonlinear summation of their inputs. The resulting model, which has a very small number of free parameters, can simulate many of the diverse properties of MT neurons. In particular, we simulate the invariance of MT tuning curves to the orientation and length of tilted bar stimuli, as well as the accompanying temporal dynamics. We also show how this property relates to the continuum from component to pattern selectivity observed when MT neurons are tested with plaids. Finally, we confirm several key predictions of the model by recording from MT neurons in the alert macaque monkey. Overall our results demonstrate that many of the seemingly complex computations carried out by high-level cortical neurons can in principle be understood by examining the properties of their inputs.
Figures





Similar articles
-
Direction and orientation selectivity of neurons in visual area MT of the macaque.J Neurophysiol. 1984 Dec;52(6):1106-30. doi: 10.1152/jn.1984.52.6.1106. J Neurophysiol. 1984. PMID: 6520628
-
Diverse suppressive influences in area MT and selectivity to complex motion features.J Neurosci. 2013 Oct 16;33(42):16715-28. doi: 10.1523/JNEUROSCI.0203-13.2013. J Neurosci. 2013. PMID: 24133273 Free PMC article.
-
Properties of pattern and component direction-selective cells in area MT of the macaque.J Neurophysiol. 2016 Jun 1;115(6):2705-20. doi: 10.1152/jn.00639.2014. Epub 2015 Nov 11. J Neurophysiol. 2016. PMID: 26561603 Free PMC article.
-
A neural-based code for computing image velocity from small sets of middle temporal (MT/V5) neuron inputs.J Vis. 2012 Aug 1;12(8):1. doi: 10.1167/12.8.1. J Vis. 2012. PMID: 22854102 Review.
-
Delineating extrastriate visual area MT(V5) using cortical myeloarchitecture.Neuroimage. 2014 Jun;93 Pt 2:231-6. doi: 10.1016/j.neuroimage.2013.03.034. Epub 2013 Mar 26. Neuroimage. 2014. PMID: 23541801 Review.
Cited by
-
Analysis on the Effect of Wushu Project Propagation in Nonmaterial Cultural Field of Environmental Protection Based on Artificial Intelligence Analysis Technology.J Environ Public Health. 2022 Oct 4;2022:9138516. doi: 10.1155/2022/9138516. eCollection 2022. J Environ Public Health. 2022. Retraction in: J Environ Public Health. 2023 Oct 18;2023:9798079. doi: 10.1155/2023/9798079. PMID: 36238822 Free PMC article. Retracted.
-
Hierarchical processing of complex motion along the primate dorsal visual pathway.Proc Natl Acad Sci U S A. 2012 Apr 17;109(16):E972-80. doi: 10.1073/pnas.1115685109. Epub 2012 Jan 31. Proc Natl Acad Sci U S A. 2012. PMID: 22308392 Free PMC article.
-
Visual motion integration by neurons in the middle temporal area of a New World monkey, the marmoset.J Physiol. 2011 Dec 1;589(Pt 23):5741-58. doi: 10.1113/jphysiol.2011.213520. Epub 2011 Sep 26. J Physiol. 2011. PMID: 21946851 Free PMC article.
-
Texture-dependent motion signals in primate middle temporal area.J Physiol. 2013 Nov 15;591(22):5671-90. doi: 10.1113/jphysiol.2013.257568. Epub 2013 Sep 2. J Physiol. 2013. PMID: 24000175 Free PMC article.
-
A Motion-from-Form Mechanism Contributes to Extracting Pattern Motion from Plaids.J Neurosci. 2016 Apr 6;36(14):3903-18. doi: 10.1523/JNEUROSCI.3398-15.2016. J Neurosci. 2016. PMID: 27053199 Free PMC article.
References
-
- Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2: 284–299, 1985. - PubMed
-
- Adelson EH, Movshon JA. Phenomenal coherence of moving visual patterns. Nature 300: 523–525, 1982. - PubMed
-
- Albrecht DG. Visual cortex neurons in monkey and cat: effect of contrast on the spatial and temporal phase transfer functions. Vis Neurosci 12: 1191–1210, 1995. - PubMed
-
- Albright TD. Direction and orientation selectivity of neurons in visual area MT of the macaque. J Neurophysiol 52: 1106–1130, 1984. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

