Retinal synaptic pathways underlying the response of the rabbit local edge detector
- PMID: 20457864
- PMCID: PMC2867570
- DOI: 10.1152/jn.00987.2009
Retinal synaptic pathways underlying the response of the rabbit local edge detector
Abstract
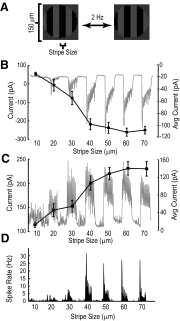
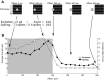
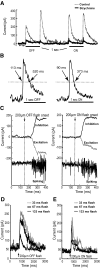
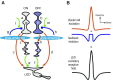
We studied the circuitry that underlies the behavior of the local edge detector (LED) retinal ganglion cell in rabbit by measuring the spatial and temporal properties of excitatory and inhibitory currents under whole cell voltage clamp. Previous work showed that LED excitation is suppressed by activity in the surround. However, the contributions of outer and inner retina to this characteristic and the neurotransmitters used are currently unknown. Blockage of retinal inhibitory pathways (GABA(A), GABA(C), and glycine) eliminated edge selectivity. Inverting gratings in the surround with 50-microm stripe sizes did not stimulate horizontal cells, but suppressed on and off excitation by roughly 60%, indicating inhibition of bipolar terminals (feedback inhibition). On pharmacologic blockage, we showed that feedback inhibition used both GABA(A) and GABA(C) receptors, but not glycine. Glycinergic inhibition suppressed GABAergic feedback inhibition in the center, enabling larger excitatory currents in response to luminance changes. Excitation, feedback inhibition, and direct (feedforward) inhibition responded to luminance-neutral flipping gratings of 20- to 50-microm widths, showing they are driven by independent subunits within their receptive fields, which confers sensitivity to borders between areas of texture and nontexture. Feedforward inhibition was glycinergic, its rise time was faster than decay time, and did not function to delay spiking at the onset of a stimulus. Both the on and off phases could be triggered by luminance shifts as short in duration as 33 ms and could be triggered during scenes that already produced a high baseline level of feedforward inhibition. Our results show how LED circuitry can use subreceptive field sensitivity to detect visual edges via the interaction between excitation and feedback inhibition and also respond to rapid luminance shifts within a rapidly changing scene by producing feedforward inhibition.
Figures





Similar articles
-
The Synaptic and Morphological Basis of Orientation Selectivity in a Polyaxonal Amacrine Cell of the Rabbit Retina.J Neurosci. 2015 Sep 30;35(39):13336-50. doi: 10.1523/JNEUROSCI.1712-15.2015. J Neurosci. 2015. PMID: 26424882 Free PMC article.
-
Synaptic currents generating the inhibitory surround of ganglion cells in the mammalian retina.J Neurosci. 2001 Jul 1;21(13):4852-63. doi: 10.1523/JNEUROSCI.21-13-04852.2001. J Neurosci. 2001. PMID: 11425912 Free PMC article.
-
Interneuron circuits tune inhibition in retinal bipolar cells.J Neurophysiol. 2010 Jan;103(1):25-37. doi: 10.1152/jn.00458.2009. Epub 2009 Nov 11. J Neurophysiol. 2010. PMID: 19906884 Free PMC article.
-
Multiple pathways of inhibition shape bipolar cell responses in the retina.Vis Neurosci. 2011 Jan;28(1):95-108. doi: 10.1017/S0952523810000209. Epub 2010 Oct 8. Vis Neurosci. 2011. PMID: 20932357 Free PMC article. Review.
-
GABAC receptor-mediated inhibition in the retina.Vision Res. 2004 Dec;44(28):3289-96. doi: 10.1016/j.visres.2004.07.023. Vision Res. 2004. PMID: 15535996 Review.
Cited by
-
Functional Circuitry of the Retina.Annu Rev Vis Sci. 2015 Nov 24;1:263-289. doi: 10.1146/annurev-vision-082114-035334. Annu Rev Vis Sci. 2015. PMID: 28532365 Free PMC article.
-
GABAergic neurotransmission and retinal ganglion cell function.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015 Mar;201(3):261-83. doi: 10.1007/s00359-015-0981-z. Epub 2015 Feb 6. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2015. PMID: 25656810 Review.
-
The Synaptic and Morphological Basis of Orientation Selectivity in a Polyaxonal Amacrine Cell of the Rabbit Retina.J Neurosci. 2015 Sep 30;35(39):13336-50. doi: 10.1523/JNEUROSCI.1712-15.2015. J Neurosci. 2015. PMID: 26424882 Free PMC article.
-
Broad thorny ganglion cells: a candidate for visual pursuit error signaling in the primate retina.J Neurosci. 2015 Apr 1;35(13):5397-408. doi: 10.1523/JNEUROSCI.4369-14.2015. J Neurosci. 2015. PMID: 25834063 Free PMC article.
-
Response Properties of a Newly Identified Tristratified Narrow Field Amacrine Cell in the Mouse Retina.PLoS One. 2015 Sep 9;10(9):e0137702. doi: 10.1371/journal.pone.0137702. eCollection 2015. PLoS One. 2015. PMID: 26352594 Free PMC article.
References
-
- Berson DM, Pu M, Famiglietti EV. The zeta cell: a new ganglion cell type in cat retina. J Comp Neurol 399: 269–288, 1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

