Circadian-independent cell mitosis in immortalized fibroblasts
- PMID: 20457900
- PMCID: PMC2906903
- DOI: 10.1073/pnas.0914078107
Circadian-independent cell mitosis in immortalized fibroblasts
Abstract
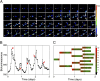
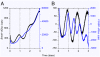
Two prominent timekeeping systems, the cell cycle, which controls cell division, and the circadian system, which controls 24-h rhythms of physiology and behavior, are found in nearly all living organisms. A distinct feature of circadian rhythms is that they are temperature-compensated such that the period of the rhythm remains constant (approximately 24 h) at different ambient temperatures. Even though the speed of cell division, or growth rate, is highly temperature-dependent, the cell-mitosis rhythm is temperature-compensated. Twenty-four-hour fluctuations in cell division have also been observed in numerous species, suggesting that the circadian system is regulating the timing of cell division. We tested whether the cell-cycle rhythm was coupled to the circadian system in immortalized rat-1 fibroblasts by monitoring cell-cycle gene promoter-driven luciferase activity. We found that there was no consistent phase relationship between the circadian and cell cycles, and that the cell-cycle rhythm was not temperature-compensated in rat-1 fibroblasts. These data suggest that the circadian system does not regulate the cell-mitosis rhythm in rat-1 fibroblasts. These findings are inconsistent with numerous studies that suggest that cell mitosis is regulated by the circadian system in mammalian tissues in vivo. To account for this discrepancy, we propose two possibilities: (i) There is no direct coupling between the circadian rhythm and cell cycle but the timing of cell mitosis is synchronized with the rhythmic host environment, or (ii) coupling between the circadian rhythm and cell cycle exists in normal cells but it is disconnected in immortalized cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
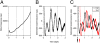
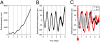
References
-
- Pittendrigh CS. Temporal organization: Reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. - PubMed
-
- Edmunds LN. Cellular and Molecular Bases of Biological Clocks: Models and Mechanisms for Circadian Timekeeping. New York: Springer; 1988.
-
- Brown WR. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol. 1991;97:273–280. - PubMed
-
- Smaaland R. Circadian rhythm of cell division. Prog Cell Cycle Res. 1996;2:241–266. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

