Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy
- PMID: 20457905
- PMCID: PMC2890766
- DOI: 10.1073/pnas.0914076107
Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy
Abstract
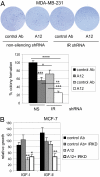
The type 1 insulin-like growth factor receptor (IGF-1R) tyrosine kinase is an important mediator of the protumorigenic effects of IGF-I/II, and inhibitors of IGF-1R signaling are currently being tested in clinical cancer trials aiming to assess the utility of this receptor as a therapeutic target. Despite mounting evidence that the highly homologous insulin receptor (IR) can also convey protumorigenic signals, its direct role in cancer progression has not been genetically defined in vivo, and it remains unclear whether such a role for IR signaling could compromise the efficacy of selective IGF-1R targeting strategies. A transgenic mouse model of pancreatic neuroendocrine carcinogenesis engages the IGF signaling pathway, as revealed by its dependence on IGF-II and by accelerated malignant progression upon IGF-1R overexpression. Surprisingly, preclinical trials with an inhibitory monoclonal antibody to IGF-1R did not significantly impact tumor growth, prompting us to investigate the involvement of IR. The levels of IR were found to be significantly up-regulated during multistep progression from hyperplastic lesions to islet tumors. Its functional involvement was revealed by genetic disruption of the IR gene in the oncogene-expressing pancreatic beta cells, which resulted in reduced tumor burden accompanied by increased apoptosis. Notably, the IR knockout tumors now exhibited sensitivity to anti-IGF-1R therapy; similarly, high IR to IGF-1R ratios demonstrably conveyed resistance to IGF-1R inhibition in human breast cancer cells. The results predict that elevated IR signaling before and after treatment will respectively manifest intrinsic and adaptive resistance to anti-IGF-1R therapies.
Conflict of interest statement
Conflict of interest statement: D.L.L. is an employee of ImClone. However, this study does not promote the ImClone drug A12.
Figures


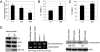
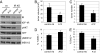
Comment in
-
An unhelpful hand.Nat Rev Cancer. 2010 Jul;10(7):453. doi: 10.1038/nrc2883. Nat Rev Cancer. 2010. PMID: 20589970 No abstract available.
-
Anticancer drugs: An unhelpful hand.Nat Rev Drug Discov. 2010 Jul;9(7):518. doi: 10.1038/nrd3211. Nat Rev Drug Discov. 2010. PMID: 20592746 No abstract available.
References
-
- Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: Overview and recent insights. Endocr Rev. 2007;28:20–47. - PubMed
-
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. - PubMed
-
- Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev. 2000;21:215–244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

