Dynamics of an emerging disease drive large-scale amphibian population extinctions
- PMID: 20457913
- PMCID: PMC2906868
- DOI: 10.1073/pnas.0914111107
Dynamics of an emerging disease drive large-scale amphibian population extinctions
Abstract
Epidemiological theory generally suggests that pathogens will not cause host extinctions because the pathogen should fade out when the host population is driven below some threshold density. An emerging infectious disease, chytridiomycosis, caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd) is directly linked to the recent extinction or serious decline of hundreds of amphibian species. Despite continued spread of this pathogen into uninfected areas, the dynamics of the host-pathogen interaction remain unknown. We use fine-scale spatiotemporal data to describe (i) the invasion and spread of Bd through three lake basins, each containing multiple populations of the mountain yellow-legged frog, and (ii) the accompanying host-pathogen dynamics. Despite intensive sampling, Bd was not detected on frogs in study basins until just before epidemics began. Following Bd arrival in a basin, the disease spread to neighboring populations at approximately 700 m/yr in a wave-like pattern until all populations were infected. Within a population, infection prevalence rapidly reached 100% and infection intensity on individual frogs increased in parallel. Frog mass mortality began only when infection intensity reached a critical threshold and repeatedly led to extinction of populations. Our results indicate that the high growth rate and virulence of Bd allow the near-simultaneous infection and buildup of high infection intensities in all host individuals; subsequent host population crashes therefore occur before Bd is limited by density-dependent factors. Preventing infection intensities in host populations from reaching this threshold could provide an effective strategy to avoid the extinction of susceptible amphibian species in the wild.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

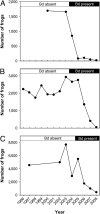
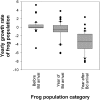

Comment in
-
It’s not easy being green.Nat Rev Microbiol. 2010 Jul;8(7):467. doi: 10.1038/nrmicro2388. Nat Rev Microbiol. 2010. PMID: 21394962 No abstract available.
References
-
- Anderson RM, May RM. Population biology of infectious diseases: Part I. Nature. 1979;280:361–367. - PubMed
-
- de Castro F, Bolker B. Mechanisms of disease-induced extinction. Ecol Lett. 2005;8:117–126.
-
- Stuart SN, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. - PubMed
-
- Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134.
-
- McCallum H. Inconclusiveness of chytridiomycosis as the agent in widespread frog declines. Conserv Biol. 2005;19:1421–1430.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

