Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians
- PMID: 20457916
- PMCID: PMC2906864
- DOI: 10.1073/pnas.0912886107
Enzootic and epizootic dynamics of the chytrid fungal pathogen of amphibians
Abstract
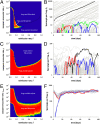
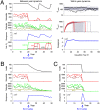
Chytridiomycosis, the disease caused by the chytrid fungus, Batrachochytrium dendrobatidis (Bd), has contributed to amphibian population declines and extinctions worldwide. The impact of this pathogen, however, varies markedly among amphibian species and populations. Following invasion into some areas of California's Sierra Nevada, Bd leads to rapid declines and local extinctions of frog populations (Rana muscosa, R. sierrae). In other areas, infected populations of the same frog species have declined but persisted at low host densities for many years. We present results of a 5-year study showing that infected adult frogs in persistent populations have low fungal loads, are surviving between years, and frequently lose and regain the infection. Here we put forward the hypothesis that fungal load dynamics can explain the different population-level outcomes of Bd observed in different areas of the Sierra Nevada and possibly throughout the world. We develop a model that incorporates the biological details of the Bd-host interaction. Importantly, model results suggest that host persistence versus extinction does not require differences in host susceptibility, pathogen virulence, or environmental conditions, and may be just epidemic and endemic population dynamics of the same host-pathogen system. The different disease outcomes seen in natural populations may result solely from density-dependent host-pathogen dynamics. The model also shows that persistence of Bd is enhanced by the long-lived tadpole stage that characterize these two frog species, and by nonhost Bd reservoirs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
It’s not easy being green.Nat Rev Microbiol. 2010 Jul;8(7):467. doi: 10.1038/nrmicro2388. Nat Rev Microbiol. 2010. PMID: 21394962 No abstract available.
References
-
- Gascon C, et al., editors. Gland, Switzerland: World Conservation Union; 2007. Amphibian Conservation Action Plan. Proceedings of the IUCN.SSC Amphibian Conservation Summit 2005. Available at http://intranet.iucn.org/webfiles/doc/SSC/SSCwebsite/GAA/ACAP_Summit_Dec.... Accessed November 1, 2009.
-
- Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227.
-
- Rachowicz LJ, et al. The novel and endemic pathogen hypotheses: Competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448.
-
- Skerratt LF, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

