Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane
- PMID: 20457922
- PMCID: PMC2906848
- DOI: 10.1073/pnas.1004515107
Sugar binding induces the same global conformational change in purified LacY as in the native bacterial membrane
Abstract
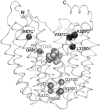
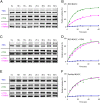
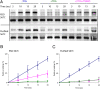
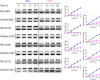
Many independent lines of evidence indicate that the lactose permease of Escherichia coli (LacY) is highly dynamic and that sugar binding causes closing of a large inward-facing cavity with opening of a wide outward-facing hydrophilic cavity. Therefore, lactose/H(+) symport catalyzed by LacY very likely involves a global conformational change that allows alternating access of single sugar- and H(+)-binding sites to either side of the membrane (the alternating access model). The x-ray crystal structures of LacY, as well as the majority of spectroscopic studies, use purified protein in detergent micelles. By using site-directed alkylation, we now demonstrate that sugar binding induces virtually the same global conformational change in LacY whether the protein is in the native bacterial membrane or is solubilized and purified in detergent. The results also indicate that the x-ray crystal structure reflects the structure of wild-type LacY in the native membrane in the absence of sugar.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Lactose permease and the alternating access mechanism.Biochemistry. 2011 Nov 15;50(45):9684-93. doi: 10.1021/bi2014294. Epub 2011 Oct 19. Biochemistry. 2011. PMID: 21995338 Free PMC article.
-
It takes two to tango: The dance of the permease.J Gen Physiol. 2019 Jul 1;151(7):878-886. doi: 10.1085/jgp.201912377. Epub 2019 May 30. J Gen Physiol. 2019. PMID: 31147449 Free PMC article. Review.
-
The alternating access transport mechanism in LacY.J Membr Biol. 2011 Jan;239(1-2):85-93. doi: 10.1007/s00232-010-9327-5. Epub 2010 Dec 16. J Membr Biol. 2011. PMID: 21161516 Free PMC article. Review.
-
Real-time conformational changes in LacY.Proc Natl Acad Sci U S A. 2014 Jun 10;111(23):8440-5. doi: 10.1073/pnas.1408374111. Epub 2014 May 28. Proc Natl Acad Sci U S A. 2014. PMID: 24872451 Free PMC article.
-
Opening the periplasmic cavity in lactose permease is the limiting step for sugar binding.Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15147-51. doi: 10.1073/pnas.1112157108. Epub 2011 Sep 6. Proc Natl Acad Sci U S A. 2011. PMID: 21896727 Free PMC article.
Cited by
-
Utilizing Biotinylated Proteins Expressed in Yeast to Visualize DNA-Protein Interactions at the Single-Molecule Level.Front Microbiol. 2017 Oct 24;8:2062. doi: 10.3389/fmicb.2017.02062. eCollection 2017. Front Microbiol. 2017. PMID: 29123507 Free PMC article.
-
Lactose permease and the alternating access mechanism.Biochemistry. 2011 Nov 15;50(45):9684-93. doi: 10.1021/bi2014294. Epub 2011 Oct 19. Biochemistry. 2011. PMID: 21995338 Free PMC article.
-
Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation.Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12420-12425. doi: 10.1073/pnas.1615414113. Epub 2016 Oct 19. Proc Natl Acad Sci U S A. 2016. PMID: 27791182 Free PMC article.
-
It takes two to tango: The dance of the permease.J Gen Physiol. 2019 Jul 1;151(7):878-886. doi: 10.1085/jgp.201912377. Epub 2019 May 30. J Gen Physiol. 2019. PMID: 31147449 Free PMC article. Review.
-
Trp replacements for tightly interacting Gly-Gly pairs in LacY stabilize an outward-facing conformation.Proc Natl Acad Sci U S A. 2013 May 28;110(22):8876-81. doi: 10.1073/pnas.1306849110. Epub 2013 May 13. Proc Natl Acad Sci U S A. 2013. PMID: 23671103 Free PMC article.
References
-
- Viitanen P, Newman MJ, Foster DL, Wilson TH, Kaback HR. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. - PubMed
-
- Menick DR, Sarkar HK, Poonian MS, Kaback HR. cys154 Is important for lac permease activity in Escherichia coli. Biochem Biophys Res Commun. 1985;132:162–170. - PubMed
-
- Smirnova IN, Kaback HR. A mutation in the lactose permease of Escherichia coli that decreases conformational flexibility and increases protein stability. Biochemistry. 2003;42:3025–3031. - PubMed
-
- Ermolova NV, Smirnova IN, Kasho VN, Kaback HR. Interhelical packing modulates conformational flexibility in the lactose permease of Escherichia coli. Biochemistry. 2005;44:7669–7677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

