Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury
- PMID: 20458011
- PMCID: PMC2919223
- DOI: 10.1161/CIRCULATIONAHA.109.928796
Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury
Abstract
Background: Understanding the mechanisms of repair and regeneration of the kidney after injury is of great interest because there are currently no therapies that promote repair, and kidneys frequently do not repair adequately. We studied the capacity of human CD34(+) hematopoietic stem/progenitor cells (HSPCs) to promote kidney repair and regeneration using an established ischemia/reperfusion injury model in mice, with particular focus on the microvasculature.
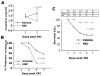
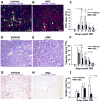
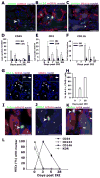
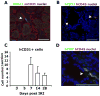
Methods and results: Human HSPCs administered systemically 24 hours after kidney injury were selectively recruited to injured kidneys of immunodeficient mice (Jackson Labs, Bar Harbor, Me) and localized prominently in and around vasculature. This recruitment was associated with enhanced repair of the kidney microvasculature, tubule epithelial cells, enhanced functional recovery, and increased survival. HSPCs recruited to kidney expressed markers consistent with circulating endothelial progenitors and synthesized high levels of proangiogenic cytokines, which promoted proliferation of both endothelial and epithelial cells. Although purified HSPCs acquired endothelial progenitor markers once recruited to the kidney, engraftment of human endothelial cells in the mouse capillary walls was an extremely rare event, indicating that human stem cell mediated renal repair is by paracrine mechanisms rather than replacement of vasculature.
Conclusions: These studies advance human HSPCs as a promising therapeutic strategy for promoting renal repair after injury.
Figures


References
-
- Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. - PubMed
-
- Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. - PubMed
-
- Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. - PubMed
-
- Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. - PubMed
-
- Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P. Stem-cell approaches for kidney repair: choosing the right cells. Trends Mol Med. 2008;14:277–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

