Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer
- PMID: 20458051
- PMCID: PMC2982784
- DOI: 10.1200/JCO.2009.25.9549
Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer
Abstract
Purpose: Retrospective studies suggest that primary breast cancers lacking estrogen receptor (ER) and progesterone receptor (PR) and not overexpressing human epidermal growth factor receptor 2 (HER2; triple-negative tumors) are particularly sensitive to DNA-damaging chemotherapy with alkylating agents.
Patients and methods: Patients enrolled in International Breast Cancer Study Group Trials VIII and IX with node-negative, operable breast cancer and centrally assessed ER, PR, and HER2 were included (n = 2,257). The trials compared three or six courses of adjuvant classical cyclophosphamide, methotrexate, and fluorouracil (CMF) with or without endocrine therapy versus endocrine therapy alone. We explored patterns of recurrence by treatment according to three immunohistochemically defined tumor subtypes: triple negative, HER2 positive and endocrine receptor absent, and endocrine receptor present.
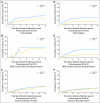
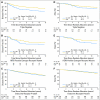
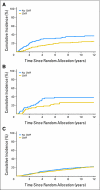
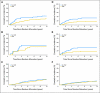
Results: Patients with triple-negative tumors (303 patients; 13%) were significantly more likely to have tumors > 2 cm and grade 3 compared with those in the HER2-positive, endocrine receptor-absent, and endocrine receptor-present subtypes. No clear chemotherapy benefit was observed in endocrine receptor-present disease (hazard ratio [HR], 0.90; 95% CI, 0.74 to 1.11). A statistically significantly greater benefit for chemotherapy versus no chemotherapy was observed in triple-negative breast cancer (HR, 0.46; 95% CI, 0.29 to 0.73; interaction P = .009 v endocrine receptor-present disease). The magnitude of the chemotherapy effect was lower in HER2-positive endocrine receptor-absent disease (HR, 0.58; 95% CI, 0.29 to 1.17; interaction P = .24 v endocrine receptor-present disease).
Conclusion: The magnitude of benefit of CMF chemotherapy is largest in patients with triple-negative, node-negative breast cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

