Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7
- PMID: 20458068
- PMCID: PMC2875925
- DOI: 10.1212/WNL.0b013e3181dd43bf
Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7
Abstract
Background: Mutations in the postsynaptic adaptor protein Dok-7 underlie congenital myasthenic syndrome (CMS) with a characteristic limb girdle pattern of muscle weakness. Patients usually do not respond to or worsen with the standard CMS treatments: cholinesterase inhibitors and 3,4-diaminopyridine. However, anecdotal reports suggest they may improve with ephedrine.
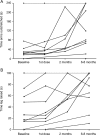
Methods: This was an open prospective follow-up study to determine muscle strength in response to ephedrine in Dok-7 CMS. Patients were first evaluated as inpatients for suitability for a trial of treatment with ephedrine. The response was assessed at 2 and 6 to 8 months follow-up clinic visits using a quantitative myasthenia gravis (severity) score (QMG) and mobility measures.
Results: Ten out of 12 of the cohort with DOK7 mutations tolerated ephedrine. We noted a progressive response to treatment over the 6 to 8 months assessment period with a significant improvement at the final QMG score (p = 0.009). Mobility scores also improved (p = 0.0006). Improvements in the subcomponents of the QMG score that measured proximal muscle function (those muscle groups most severely affected) were most marked, and in some cases were dramatic. All patients reported enhanced activities of daily living at 6-8 months.
Conclusion: Ephedrine appears to be an effective treatment for Dok-7 CMS. It is well-tolerated by most patients and improvement in strength can be profound. Determining the long-term response and the most effective dosing regimen will require further research.
Classification of evidence: This study provides Class IV evidence that ephedrine given at doses between 15 and 90 mg/day improves muscle strength in patients with documented mutations in DOK7.
Figures



References
-
- Palace J, Beeson D. The congenital myasthenic syndromes. J Neuroimmunol 2008;201–202:2–5. - PubMed
-
- Beeson D, Higuchi O, Palace J, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 2006;313:1975–1978. - PubMed
-
- Muller J, Herczegfalvi A, Vilchez J, et al. Phenotypical spectrum of DOK7 mutations in congenital myasthenic syndromes. Brain 2007;130:1497–1506. - PubMed
-
- Palace J, Lashley D, Newsom-Davis J, et al. Clinical features of the DOK7 neuromuscular junction synaptopathy. Brain 2007;130:1507–1515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
