Biochemical characterization of cholesteryl ester transfer protein inhibitors
- PMID: 20458119
- PMCID: PMC2918456
- DOI: 10.1194/jlr.M007468
Biochemical characterization of cholesteryl ester transfer protein inhibitors
Abstract
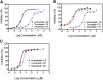
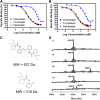
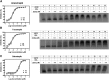
Cholesteryl ester transfer protein (CETP) has been identified as a novel target for increasing HDL cholesterol levels. In this report, we describe the biochemical characterization of anacetrapib, a potent inhibitor of CETP. To better understand the mechanism by which anacetrapib inhibits CETP activity, its biochemical properties were compared with CETP inhibitors from distinct structural classes, including torcetrapib and dalcetrapib. Anacetrapib and torcetrapib inhibited CETP-mediated cholesteryl ester and triglyceride transfer with similar potencies, whereas dalcetrapib was a significantly less potent inhibitor. Inhibition of CETP by both anacetrapib and torcetrapib was not time dependent, whereas the potency of dalcetrapib significantly increased with extended preincubation. Anacetrapib, torcetrapib, and dalcetrapib compete with one another for binding CETP; however anacetrapib binds reversibly and dalcetrapib covalently to CETP. In addition, dalcetrapib was found to covalently label both human and mouse plasma proteins. Each CETP inhibitor induced tight binding of CETP to HDL, indicating that these inhibitors promote the formation of a complex between CETP and HDL, resulting in inhibition of CETP activity.
Figures




Similar articles
-
Cholesteryl ester transfer-protein modulator and inhibitors and their potential for the treatment of cardiovascular diseases.Vasc Health Risk Manag. 2012;8:323-31. doi: 10.2147/VHRM.S25238. Epub 2012 May 15. Vasc Health Risk Manag. 2012. PMID: 22661899 Free PMC article. Review.
-
Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective.Clin Pharmacokinet. 2013 Aug;52(8):615-26. doi: 10.1007/s40262-013-0071-8. Clin Pharmacokinet. 2013. PMID: 23658137 Free PMC article.
-
Assessing the mechanisms of cholesteryl ester transfer protein inhibitors.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Dec;1862(12):1606-1617. doi: 10.1016/j.bbalip.2017.09.004. Epub 2017 Sep 12. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28911944 Free PMC article.
-
Modulating cholesteryl ester transfer protein activity maintains efficient pre-β-HDL formation and increases reverse cholesterol transport.J Lipid Res. 2010 Dec;51(12):3443-54. doi: 10.1194/jlr.M008706. Epub 2010 Sep 22. J Lipid Res. 2010. PMID: 20861162 Free PMC article.
-
Different effects of compounds decreasing cholesteryl ester transfer protein activity on lipoprotein metabolism.Curr Opin Lipidol. 2011 Aug;22(4):288-95. doi: 10.1097/MOL.0b013e3283475e00. Curr Opin Lipidol. 2011. PMID: 21587074 Review.
Cited by
-
Clinical Pharmacokinetics and Pharmacodynamics of Dalcetrapib.Clin Pharmacokinet. 2018 Nov;57(11):1359-1367. doi: 10.1007/s40262-018-0656-3. Clin Pharmacokinet. 2018. PMID: 29730761 Free PMC article. Review.
-
Cholesteryl ester transfer protein and its inhibitors.J Lipid Res. 2018 May;59(5):772-783. doi: 10.1194/jlr.R082735. Epub 2018 Feb 27. J Lipid Res. 2018. PMID: 29487091 Free PMC article. Review.
-
New horizons for cholesterol ester transfer protein inhibitors.Curr Atheroscler Rep. 2012 Feb;14(1):41-8. doi: 10.1007/s11883-011-0217-9. Curr Atheroscler Rep. 2012. PMID: 22083134 Review.
-
Evaluation of CETP activity in vivo under non-steady-state conditions: influence of anacetrapib on HDL-TG flux.J Lipid Res. 2016 Mar;57(3):398-409. doi: 10.1194/jlr.M063842. Epub 2015 Dec 9. J Lipid Res. 2016. PMID: 26658238 Free PMC article.
-
Indolinyl-Thiazole Based Inhibitors of Scavenger Receptor-BI (SR-BI)-Mediated Lipid Transport.ACS Med Chem Lett. 2015 Feb 2;6(4):375-380. doi: 10.1021/ml500154q. eCollection 2015 Apr 9. ACS Med Chem Lett. 2015. PMID: 26478787 Free PMC article.
References
-
- Gordon D. J., Probstfield J. L., Garrison R. J., Neaton J. D., Castelli W. P., Knoke J. D., Jacobs D. R., Bangdiwala S., Tyroler H. A. 1989. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 79: 8–15. - PubMed
-
- Assmann G., Schulte H., Eckardstein A., Huang Y. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 124: S11–S20. - PubMed
-
- Sharrett A. R., Ballantyne C. M., Coady S. A., Heiss G., Sorlie P. D., Catellier D., Patsch W. 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 104: 1108–1113. - PubMed
-
- Luc G., Bard J., Ferrieres J., Evans A., Amouyel P., Arveiler D., Fruchart J., Ducimetiere P., on behalf of the PRIME Study Group. 2002. Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: The PRIME Study. Arterioscler. Thromb. Vasc. Biol. 22: 1155–1161. - PubMed
-
- Boden W. E. 2000. High-density lipoprotein cholesterol as an independent risk factor in cardiovascular disease: assessing the data from Framingham to the Veterans Affairs High–Density Lipoprotein Intervention Trial. Am. J. Cardiol. 86: 19L–22L. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

