Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation
- PMID: 20458140
- PMCID: PMC2877924
- DOI: 10.1172/JCI37646
Rapid T cell-based identification of human tumor tissue antigens by automated two-dimensional protein fractionation
Abstract
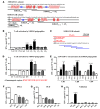
Identifying the antigens that have the potential to trigger endogenous antitumor responses in an individual cancer patient is likely to enhance the efficacy of cancer immunotherapy, but current methodologies do not efficiently identify such antigens. This study describes what we believe to be a new method of comprehensively identifying candidate tissue antigens that spontaneously cause T cell responses in disease situations. We used the newly developed automated, two-dimensional chromatography system PF2D to fractionate the proteome of human tumor tissues and tested protein fractions for recognition by preexisting tumor-specific CD4+ Th cells and CTLs. Applying this method using mice transgenic for a TCR that recognizes an OVA peptide presented by MHC class I, we demonstrated efficient separation, processing, and cross-presentation to CD8+ T cells by DCs of OVA expressed by the OVA-transfected mouse lymphoma RMA-OVA. Applying this method to human tumor tissues, we identified MUC1 and EGFR as tumor-associated antigens selectively recognized by T cells in patients with head and neck cancer. Finally, in an exemplary patient with a malignant brain tumor, we detected CD4+ and CD8+ T cell responses against two novel antigens, transthyretin and calgranulin B/S100A9, which were expressed in tumor and endothelial cells. The immunogenicity of these antigens was confirmed in 4 of 10 other brain tumor patients. This fast and inexpensive method therefore appears suitable for identifying candidate T cell antigens in various disease situations, such as autoimmune and malignant diseases, without being restricted to expression by a certain cell type or HLA allele.
Figures
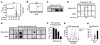





References
-
- Weinschenk T, et al. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002;62(20):5818–5827. - PubMed
-
- Celis E, et al. Identification of potential CTL epitopes of tumor-associated antigen MAGE-1 for five common HLA-A alleles. Mol Immunol. 1994;31(18):1423–1430. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

