The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b
- PMID: 20458145
- PMCID: PMC2877939
- DOI: 10.1172/JCI40380
The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b
Abstract
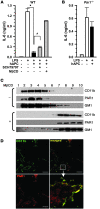
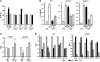
Activated protein C (APC), the only FDA-approved biotherapeutic drug for sepsis, possesses anticoagulant, antiinflammatory, and barrier-protective activities. However, the mechanisms underlying its anti-inflammatory functions are not well defined. Here, we report that the antiinflammatory activity of APC on macrophages is dependent on integrin CD11b/CD18, but not on endothelial protein C receptor (EPCR). We showed that CD11b/CD18 bound APC within specialized membrane microdomains/lipid rafts and facilitated APC cleavage and activation of protease-activated receptor-1 (PAR1), leading to enhanced production of sphingosine-1-phosphate (S1P) and suppression of the proinflammatory response of activated macrophages. Deletion of the gamma-carboxyglutamic acid domain of APC, a region critical for its anticoagulant activity and EPCR-dependent barrier protection, had no effect on its antiinflammatory function. Genetic inactivation of CD11b, PAR1, or sphingosine kinase-1, but not EPCR, abolished the ability of APC to suppress the macrophage inflammatory response in vitro. Using an LPS-induced mouse model of lethal endotoxemia, we showed that APC administration reduced the mortality of wild-type mice, but not CD11b-deficient mice. These data establish what we believe to be a novel mechanism underlying the antiinflammatory activity of APC in the setting of endotoxemia and provide clear evidence that the antiinflammatory function of APC is distinct from its barrier-protective function and anticoagulant activities.
Figures
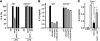



Similar articles
-
Endotoxemia and sepsis mortality reduction by non-anticoagulant activated protein C.J Exp Med. 2007 Oct 1;204(10):2439-48. doi: 10.1084/jem.20070404. Epub 2007 Sep 24. J Exp Med. 2007. PMID: 17893198 Free PMC article.
-
Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice.J Clin Invest. 2010 Sep;120(9):3167-78. doi: 10.1172/JCI42629. Epub 2010 Aug 16. J Clin Invest. 2010. PMID: 20714108 Free PMC article.
-
Protection of vascular barrier integrity by activated protein C in murine models depends on protease-activated receptor-1.Thromb Haemost. 2009 Apr;101(4):724-33. doi: 10.1160/th08-10-0632. Thromb Haemost. 2009. PMID: 19350118 Free PMC article.
-
Crosstalk between inflammation and coagulation: the lessons of sepsis.Curr Vasc Pharmacol. 2012 Sep;10(5):632-8. doi: 10.2174/157016112801784549. Curr Vasc Pharmacol. 2012. PMID: 22272914 Review.
-
The structure-function relationship of activated protein C. Lessons from natural and engineered mutations.Thromb Haemost. 2011 Dec;106(6):1034-45. doi: 10.1160/TH11-08-0522. Epub 2011 Nov 10. Thromb Haemost. 2011. PMID: 22072231 Review.
Cited by
-
Podocyte Integrin-β3 and Activated Protein C Coordinately Restrict RhoA Signaling and Ameliorate Diabetic Nephropathy.J Am Soc Nephrol. 2020 Aug;31(8):1762-1780. doi: 10.1681/ASN.2019111163. Epub 2020 Jul 24. J Am Soc Nephrol. 2020. PMID: 32709711 Free PMC article.
-
Rare variants at 16p11.2 are associated with common variable immunodeficiency.J Allergy Clin Immunol. 2015 Jun;135(6):1569-77. doi: 10.1016/j.jaci.2014.12.1939. Epub 2015 Feb 10. J Allergy Clin Immunol. 2015. PMID: 25678086 Free PMC article.
-
Phagocytosis is the main CR3-mediated function affected by the lupus-associated variant of CD11b in human myeloid cells.PLoS One. 2013;8(2):e57082. doi: 10.1371/journal.pone.0057082. Epub 2013 Feb 22. PLoS One. 2013. PMID: 23451151 Free PMC article.
-
Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway.J Thromb Haemost. 2013 Jun;11 Suppl 1(0 1):242-53. doi: 10.1111/jth.12247. J Thromb Haemost. 2013. PMID: 23809128 Free PMC article. Review.
-
Cytoprotective protein C pathways and implications for stroke and neurological disorders.Trends Neurosci. 2011 Apr;34(4):198-209. doi: 10.1016/j.tins.2011.01.005. Epub 2011 Feb 25. Trends Neurosci. 2011. PMID: 21353711 Free PMC article. Review.
References
-
- Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37(1 suppl):S30–S37. - PubMed
-
- Fukudome K, Esmon CT. Identification, cloning, and regulation of a novel endothelial cell protein C/activated protein C receptor. J Biol Chem. 1994;269(42):26486–26491. - PubMed
-
- Esmon CT, Esmon NL, Harris KW. Complex formation between thrombin and thrombomodulin inhibits both thrombin-catalyzed fibrin formation and factor V activation. J Biol Chem. 1982;257(14):7944–7947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

