Lnk constrains myeloproliferative diseases in mice
- PMID: 20458146
- PMCID: PMC2877957
- DOI: 10.1172/JCI42032
Lnk constrains myeloproliferative diseases in mice
Abstract
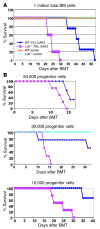
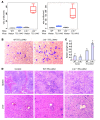
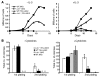
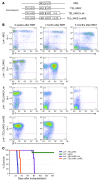
Hematopoietic stem and progenitor cell (HSPC) expansion is regulated by intrinsic signaling pathways activated by cytokines. The intracellular kinase JAK2 plays an essential role in cytokine signaling, and activating mutations in JAK2 are found in a number of hematologic malignancies. We previously demonstrated that lymphocyte adaptor protein (Lnk, also known as Sh2b3) binds JAK2 and attenuates its activity, thereby limiting HSPC expansion. Here we show that loss of Lnk accelerates and exacerbates oncogenic JAK2-induced myeloproliferative diseases (MPDs) in mice. Specifically, Lnk deficiency enhanced cytokine-independent JAK/STAT signaling and augmented the ability of oncogenic JAK2 to expand myeloid progenitors in vitro and in vivo. An activated form of JAK2, unable to bind Lnk, caused greater myeloid expansion than activated JAK2 alone and accelerated myelofibrosis, indicating that Lnk directly inhibits oncogenic JAK2 in constraining MPD development. In addition, Lnk deficiency cooperated with the BCR/ABL oncogene, the product of which does not directly interact with or depend on JAK2 or Lnk, in chronic myeloid leukemia (CML) development, suggesting that Lnk also acts through endogenous pathways to constrain HSPCs. Consistent with this idea, aged Lnk-/- mice spontaneously developed a CML-like MPD. Taken together, our data establish Lnk as a bona fide suppressor of MPD in mice and raise the possibility that Lnk dysfunction contributes to the development of hematologic malignancies in humans.
Figures


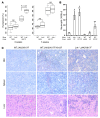
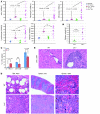
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

