Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies
- PMID: 20458189
- PMCID: PMC3180085
- DOI: 10.4161/mabs.11986
Rapid comparison of a candidate biosimilar to an innovator monoclonal antibody with advanced liquid chromatography and mass spectrometry technologies
Abstract
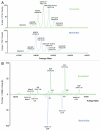
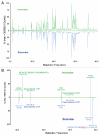
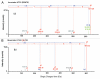
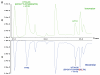
This study shows that state-of-the-art liquid chromatography (LC) and mass spectrometry (MS) can be used for rapid verification of identity and characterization of sequence variants and posttranslational modifications (PTMs) for antibody products. A candidate biosimilar IgG1 monoclonal antibody (mAb) was compared in detail to a commercially available innovator product. Intact protein mass, primary sequence, PTMs, and the micro-differences between the two mAbs were identified and quantified simultaneously. Although very similar in terms of sequences and modifications, a mass difference observed by LC-MS intact mass measurements indicated that they were not identical. Peptide mapping, performed with data independent acquisition LC-MS using an alternating low and elevated collision energy scan mode (LC-MS(E)), located the mass difference between the biosimilar and the innovator to a two amino acid residue variance in the heavy chain sequences. The peptide mapping technique was also used to comprehensively catalogue and compare the differences in PTMs of the biosimilar and innovator mAbs. Comprehensive glycosylation profiling confirmed that the proportion of individual glycans was different between the biosimilar and the innovator, although the number and identity of glycans were the same. These results demonstrate that the combination of accurate intact mass measurement, released glycan profiling, and LC-MS(E) peptide mapping provides a set of routine tools that can be used to comprehensively compare a candidate biosimilar and an innovator mAb.
Figures





Similar articles
-
[Structural characterization and analysis of Omalizumab and its biosimilar CMAB007 by LC-MS].Sheng Wu Gong Cheng Xue Bao. 2016 Apr 25;32(4):497-506. doi: 10.13345/j.cjb.150339. Sheng Wu Gong Cheng Xue Bao. 2016. PMID: 28853271 Chinese.
-
Versatile characterization of glycosylation modification in CTLA4-Ig fusion proteins by liquid chromatography-mass spectrometry.MAbs. 2014;6(6):1474-85. doi: 10.4161/mabs.36313. MAbs. 2014. PMID: 25484062 Free PMC article.
-
Monoclonal antibodies biosimilarity assessment using transient isotachophoresis capillary zone electrophoresis-tandem mass spectrometry.MAbs. 2014;6(6):1464-73. doi: 10.4161/mabs.36305. MAbs. 2014. PMID: 25484058 Free PMC article.
-
Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications.Anal Chim Acta. 2019 Dec 16;1089:1-18. doi: 10.1016/j.aca.2019.08.044. Epub 2019 Aug 22. Anal Chim Acta. 2019. PMID: 31627805 Review.
-
Qualitative and quantitative characterization of protein biotherapeutics with liquid chromatography mass spectrometry.Mass Spectrom Rev. 2017 Nov;36(6):734-754. doi: 10.1002/mas.21500. Epub 2016 Apr 20. Mass Spectrom Rev. 2017. PMID: 27097288 Review.
Cited by
-
A novel peptide design aids in the expression and its simplified process of manufacturing of Insulin Glargine in Pichia pastoris.Appl Microbiol Biotechnol. 2021 Apr;105(8):3061-3074. doi: 10.1007/s00253-021-11224-y. Epub 2021 Apr 5. Appl Microbiol Biotechnol. 2021. PMID: 33821296
-
Charge variants characterization and release assay development for co-formulated antibodies as a combination therapy.MAbs. 2019 Apr;11(3):489-499. doi: 10.1080/19420862.2019.1578137. Epub 2019 Feb 20. MAbs. 2019. PMID: 30786796 Free PMC article.
-
Glycan Profile Analysis of Engineered Trastuzumab with Rationally Added Glycosylation Sequons Presents Significantly Increased Glycan Complexity.Pharmaceutics. 2021 Oct 20;13(11):1747. doi: 10.3390/pharmaceutics13111747. Pharmaceutics. 2021. PMID: 34834161 Free PMC article.
-
Soft-sensor model development for CHO growth/production, intracellular metabolite, and glycan predictions.Front Mol Biosci. 2024 Oct 22;11:1441885. doi: 10.3389/fmolb.2024.1441885. eCollection 2024. Front Mol Biosci. 2024. PMID: 39502716 Free PMC article.
-
Rapid and multi-level characterization of trastuzumab using sheathless capillary electrophoresis-tandem mass spectrometry.MAbs. 2013 May-Jun;5(3):479-90. doi: 10.4161/mabs.23995. Epub 2013 Apr 5. MAbs. 2013. PMID: 23563524 Free PMC article.
References
-
- The Henry J. Kaiser Family Foundation U.S. Healthcare Costs, author. www.kaiseredu.org/topics_im.asp?imID=1&parentID=61&id=358.
-
- Hughes B. Gearing up for follow-on biologics. Nat Rev Drug Discov. 2009;8:181. - PubMed
-
- Questions and Answers on Biosimilar Medicines (Similar Biological Medicinal Products); London, 22 October 2008; Doc Ref EMEA/74562/2006 Rev 1. www.ema.europa.eu/pdfs/human/pcwp/7456206en.pdf.
-
- Procedures for Marketing Authorization Volume 2A, Chapter 1, November 2005. http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/vol-2/a/vol2a_ch....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
