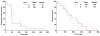Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma
- PMID: 20458210
- PMCID: PMC3030655
- DOI: 10.1097/COC.0b013e3181d2734a
Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma
Abstract
Purpose: Treatment options are limited for advanced pancreatic cancer progressive after gemcitabine therapy. The vascular endothelial growth factor pathway is biologically important in pancreatic cancer, and docetaxel has modest antitumor activity. We evaluated the role of the anti-vascular endothelial growth factor antibody bevacizumab as second-line treatment for patients with metastatic pancreatic cancer.
Design: Patients with metastatic adenocarcinoma of the pancreas who had progressive disease on a gemcitabine-containing regimen were randomized to receive bevacizumab alone or bevacizumab in combination with docetaxel.
Results: Thirty-two patients were enrolled; 16 to bevacizumab alone (Arm A) and 16 to bevacizumab plus docetaxel (Arm B). Toxicities were greater in Arm B with the most common grade 3/4 nonhematologic toxicities including fatigue, diarrhea, dehydration, and anorexia. No confirmed objective responses were observed. At 4 months, 2 of the 16 patients in Arm A and 3 of the 16 patients in Arm B were free from progression. The study was stopped according to the early stopping rule for futility. Median progression-free survival and overall survival were 43 days and 165 days in Arm A and 48 days and 125 days in Arm B. Elevated d-dimer levels and thrombin-antithrombin complexes were associated with decreased survival and increased toxicity.
Conclusion: Bevacizumab with or without docetaxel does not have antitumor activity in gemcitabine-refractory metastatic pancreatic cancer. Baseline and on-treatment d-dimer and thrombin-antithrombin complex levels are associated with increased toxicity and decreased survival.
Figures
References
-
- Burris HA, 3rd, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–13. - PubMed
-
- Moore MJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–6. - PubMed
-
- Reni M, et al. A multi-centre retrospective review of second-line therapy in advanced pancreatic adenocarcinoma. Cancer Chemother Pharmacol. 2008 - PubMed
-
- Gebbia V, et al. Second-line chemotherapy in advanced pancreatic carcinoma: a multicenter survey of the Gruppo Oncologico Italia Meridionale on the activity and safety of the FOLFOX4 regimen in clinical practice. Ann Oncol. 2007;18(Suppl 6):vi124–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical