Structure of the EGF receptor transactivation circuit integrates multiple signals with cell context
- PMID: 20458382
- PMCID: PMC3306786
- DOI: 10.1039/c003921g
Structure of the EGF receptor transactivation circuit integrates multiple signals with cell context
Abstract
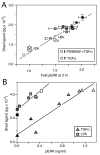
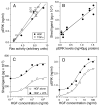
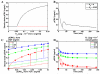
Transactivation of the epidermal growth factor receptor (EGFR) is thought to be a process by which a variety of cellular inputs can be integrated into a single signaling pathway through either stimulated proteolysis (shedding) of membrane-anchored EGFR ligands or by modification of the activity of the EGFR. As a first step towards building a predictive model of the EGFR transactivation circuit, we quantitatively defined how signals from multiple agonists were integrated both upstream and downstream of the EGFR to regulate extracellular signal regulated kinase (ERK) activity in human mammary epithelial cells. By using a "non-binding" reporter of ligand shedding, we found that transactivation triggers a positive feedback loop from ERK back to the EGFR such that ligand shedding drives EGFR-stimulated ERK that in turn drives further ligand shedding. Importantly, activated Ras and ERK levels were nearly linear functions of ligand shedding and the effect of multiple, sub-saturating inputs was additive. Simulations showed that ERK-mediated feedback through ligand shedding resulted in a stable steady-state level of activated ERK, but also showed that the extracellular environment can modulate the level of feedback. Our results suggest that the transactivation circuit acts as a context-dependent integrator and amplifier of multiple extracellular signals and that signal integration can effectively occur at multiple points in the EGFR pathway.
Figures





Similar articles
-
Multiple mechanisms are responsible for transactivation of the epidermal growth factor receptor in mammary epithelial cells.J Biol Chem. 2008 Nov 14;283(46):31477-87. doi: 10.1074/jbc.M800456200. Epub 2008 Sep 9. J Biol Chem. 2008. PMID: 18782770 Free PMC article.
-
Constitutive coupling of a naturally occurring human alpha1a-adrenergic receptor genetic variant to EGFR transactivation pathway.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19796-801. doi: 10.1073/pnas.1116271108. Epub 2011 Nov 16. Proc Natl Acad Sci U S A. 2011. PMID: 22089237 Free PMC article.
-
Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor.Invest Ophthalmol Vis Sci. 2007 Feb;48(2):636-43. doi: 10.1167/iovs.06-0203. Invest Ophthalmol Vis Sci. 2007. PMID: 17251460 Free PMC article.
-
ERK1/2 mediate wounding- and G-protein-coupled receptor ligands-induced EGFR activation via regulating ADAM17 and HB-EGF shedding.Invest Ophthalmol Vis Sci. 2009 Jan;50(1):132-9. doi: 10.1167/iovs.08-2246. Epub 2008 Jul 24. Invest Ophthalmol Vis Sci. 2009. PMID: 18658095 Free PMC article.
-
ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors.Am J Physiol Cell Physiol. 2006 Jul;291(1):C1-10. doi: 10.1152/ajpcell.00620.2005. Am J Physiol Cell Physiol. 2006. PMID: 16769815 Review.
Cited by
-
Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo.Nat Commun. 2013;4:1504. doi: 10.1038/ncomms2506. Nat Commun. 2013. PMID: 23422672 Free PMC article.
-
Cellular context alters EGF-induced ERK dynamics and reveals potential crosstalk with GDF-15.Biomicrofluidics. 2022 Oct 7;16(5):054104. doi: 10.1063/5.0114334. eCollection 2022 Sep. Biomicrofluidics. 2022. PMID: 36217350 Free PMC article.
-
Biology using engineering tools: the negative feedback amplifier.Cell Cycle. 2011 Jul 1;10(13):2069-76. doi: 10.4161/cc.10.13.16245. Epub 2011 Jul 1. Cell Cycle. 2011. PMID: 21572255 Free PMC article.
-
Data-driven modeling reconciles kinetics of ERK phosphorylation, localization, and activity states.Mol Syst Biol. 2014 Jan 30;10(1):718. doi: 10.1002/msb.134708. Print 2014. Mol Syst Biol. 2014. PMID: 24489118 Free PMC article.
-
Raf family kinases: old dogs have learned new tricks.Genes Cancer. 2011 Mar;2(3):232-60. doi: 10.1177/1947601911407323. Genes Cancer. 2011. PMID: 21779496 Free PMC article.
References
-
- Bhola NE, Grandis JR. Front Biosci. 2008;13:1857–1865. - PubMed
-
- Citri A, Yarden Y. Nat Rev Mol Cell Biol. 2006;7:505–516. - PubMed
-
- Fischer OM, Hart S, Gschwind A, Ullrich A. Biochem. Soc. Trans. 2003;31:1203–1208. - PubMed
-
- Prenzel N, Fischer OM, Streit S, Hart S, Ullrich A. Endocr. Rela.t Cancer. 2001;8:11–31. - PubMed
-
- Zwick E, Hackel PO, Prenzel N, Ullrich A. Trends Pharmacol. Sci. 1999;20:408–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

