Pentalysine beta-carbonylphthalocyanine zinc: an effective tumor-targeting photosensitizer for photodynamic therapy
- PMID: 20458713
- PMCID: PMC2935799
- DOI: 10.1002/cmdc.201000042
Pentalysine beta-carbonylphthalocyanine zinc: an effective tumor-targeting photosensitizer for photodynamic therapy
Abstract
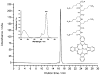
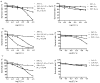
Unsymmetrical phthalocyanine derivatives have been widely studied as photosensitizers for photodynamic therapy (PDT), targeting various tumor types. However, the preparation of unsymmetrical phthalocyanines is always a challenge due to the presence of many possible structural isomers. Herein we report a new unsymmetrical zinc phthalocyanine, pentalysine beta-carbonylphthalocyanine zinc (ZnPc-(Lys)(5)), that was prepared in large quantity and high purity. This is a water-soluble cationic photosensitizer and maintains a high quantum yield of singlet oxygen generation similar to that of unsubstituted zinc phthalocyanine (ZnPc). Compared with anionic ZnPc counterparts, ZnPc-(Lys)(5) shows a higher level cellular uptake and 20-fold higher phototoxicity toward tumor cells. Pharmacokinetics and PDT studies of ZnPc-(Lys)(5) in S180 tumor-bearing mice showed a high ratio of tumor versus skin retention and significant tumor inhibition. This new molecular framework will allow synthetic diversity in the number of lysine residues incorporated and will facilitate future QSAR studies.
Figures


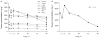

Similar articles
-
Receptor-targeting phthalocyanine photosensitizer for improving antitumor photocytotoxicity.PLoS One. 2012;7(5):e37051. doi: 10.1371/journal.pone.0037051. Epub 2012 May 31. PLoS One. 2012. PMID: 22693566 Free PMC article.
-
A Nanoencapsulated Ir(III)-Phthalocyanine Conjugate as a Promising Photodynamic Therapy Anticancer Agent.ACS Appl Mater Interfaces. 2024 Jul 31;16(30):38916-38930. doi: 10.1021/acsami.4c05181. Epub 2024 Jul 23. ACS Appl Mater Interfaces. 2024. PMID: 39041453 Free PMC article.
-
Preparation of aggregation-free ZnPc-doped nanophotosensitizers for highly efficient photodynamic therapy.Nanotechnology. 2025 Feb 18;36(13). doi: 10.1088/1361-6528/adb437. Nanotechnology. 2025. PMID: 39928992
-
Tetra-triethyleneoxysulfonyl substituted zinc phthalocyanine for photodynamic cancer therapy.Photodiagnosis Photodyn Ther. 2016 Mar;13:148-157. doi: 10.1016/j.pdpdt.2015.07.001. Epub 2015 Jul 7. Photodiagnosis Photodyn Ther. 2016. PMID: 26162500 Review.
-
Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy.J Pharm Biomed Anal. 2014 Jan;87:98-104. doi: 10.1016/j.jpba.2013.05.014. Epub 2013 May 18. J Pharm Biomed Anal. 2014. PMID: 23746989 Review.
Cited by
-
Expanding the applications of photodynamic therapy-tooth bleaching.Clin Oral Investig. 2022 Feb;26(2):2175-2186. doi: 10.1007/s00784-021-04199-7. Epub 2021 Oct 16. Clin Oral Investig. 2022. PMID: 34657223
-
Dental Bleaching with Phthalocyanine Photosensitizers: Effects on Dentin Color and Collagen Content.Molecules. 2023 May 22;28(10):4223. doi: 10.3390/molecules28104223. Molecules. 2023. PMID: 37241963 Free PMC article.
-
Investigations on the antitumor activity of classical trifluoro-substituted zinc phthalocyanines derivatives.World J Microbiol Biotechnol. 2018 Mar 17;34(4):52. doi: 10.1007/s11274-018-2422-y. World J Microbiol Biotechnol. 2018. PMID: 29550886
-
Monomer and Oligomer Transition of Zinc Phthalocyanine Is Key for Photobleaching in Photodynamic Therapy.Molecules. 2023 Jun 8;28(12):4639. doi: 10.3390/molecules28124639. Molecules. 2023. PMID: 37375194 Free PMC article.
-
Potent inhibition of Severe Acute Respiratory Syndrome Coronavirus 2 by photosensitizers compounds.Dyes Pigm. 2021 Oct;194:109570. doi: 10.1016/j.dyepig.2021.109570. Epub 2021 Jun 22. Dyes Pigm. 2021. PMID: 34183871 Free PMC article.
References
-
- Hamblin MR, Mroz P, editors. Advances in Photodynamic Therapy: Basic, Translational and Clinical. Artech House; Norwood: 2008.
-
- Brown SB, Brown EA, Walker I. Lancet Oncol. 2004;5:497–508. - PubMed
-
- Dolmans DE, Fukumura D, Jain RK. Nat Rev Cancer. 2003;3:380–387. - PubMed
-
- Allen CM, Sharman WM, van Lier JE. J Porphyrins Phthalocyanines. 2001;05:161–169.
-
- van Lier JE, Spikes JD. In: Ciba Foundation Symposium 146 – Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use. Bock G, Harnett S, editors. Wiley; Chichester: 1989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

