Centrosome biogenesis continues in the absence of microtubules during prolonged S-phase arrest
- PMID: 20458743
- PMCID: PMC2930098
- DOI: 10.1002/jcp.22222
Centrosome biogenesis continues in the absence of microtubules during prolonged S-phase arrest
Abstract
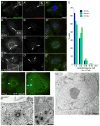
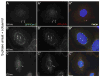
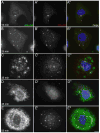
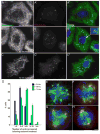
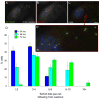
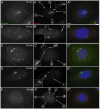
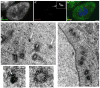
When CHO cells are arrested in S-phase, they undergo repeated rounds of centrosome duplication without cell-cycle progression. While the increase is slow and asynchronous, the number of centrosomes in these cells does rise with time. To investigate mechanisms controlling this duplication, we have arrested CHO cells in S-phase for up to 72 h, and coordinately inhibited new centriole formation by treatment with the microtubule poison colcemid. We find that in such cells, the pre-existing centrosomes remain, and a variable number of foci--containing alpha/gamma-tubulin and centrin 2--assemble at the nuclear periphery. When the colcemid is washed out, the nuclear-associated foci disappear, and cells assemble new centriole-containing centrosomes, which accumulate the centriole scaffold protein SAS-6, nucleate microtubule asters, and form functional mitotic spindle poles. The number of centrosomes that assemble following colcemid washout increases with duration of S-phase arrest, even though the number of nuclear-associated foci or pre-existing centrosomes does not increase. This suggests that during S-phase, a cryptic generative event occurs repeatedly, even in the absence of new triplet microtubule assembly. When triplet microtubule assembly is restored, these cryptic generative events become realized, and multiple centriole-containing centrosomes assemble.
(c) 2010 Wiley-Liss, Inc.
Figures

References
-
- Balczon R, Varden CE, Schroer TA. Role for microtubules in centrosome doubling in Chinese hamster ovary cells. Cell Motil Cytoskeleton. 1999;42:60–72. - PubMed
-
- Bettencourt-Dias M, Glover DM. Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol. 2007;8:451–463. - PubMed
-
- Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol. 2005;15:2199–2207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

