Soluble guanylate cyclase is activated differently by excess NO and by YC-1: resonance Raman spectroscopic evidence
- PMID: 20459051
- PMCID: PMC2883567
- DOI: 10.1021/bi100506j
Soluble guanylate cyclase is activated differently by excess NO and by YC-1: resonance Raman spectroscopic evidence
Abstract
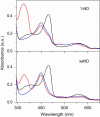
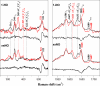
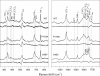
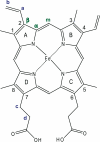
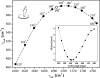
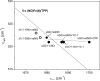
Modulation of soluble guanylate cyclase (sGC) activity by nitric oxide (NO) involves two distinct steps. Low-level activation of sGC is achieved by the stoichiometric binding of NO (1-NO) to the heme cofactor, while much higher activation is achieved by the binding of additional NO (xsNO) at a non-heme site. Addition of the allosteric activator YC-1 to the 1-NO form leads to activity comparable to that of the xsNO state. In this study, the mechanisms of sGC activation were investigated using electronic absorption and resonance Raman (RR) spectroscopic methods. RR spectroscopy confirmed that the 1-NO form contains five-coordinate NO-heme and showed that the addition of NO to the 1-NO form has no significant effect on the spectrum. In contrast, addition of YC-1 to either the 1-NO or xsNO forms alters the RR spectrum significantly, indicating a protein-induced change in the heme geometry. This change in the heme geometry was also observed when BAY 41-2272 was added to the xsNO form. Bands assigned to bending and stretching motions of the vinyl and propionate substituents undergo changes in intensity in a pattern suggesting altered tilting of the pyrrole rings to which they are attached. In addition, the N-O stretching frequency increases, with no change in the Fe-NO stretching frequency, an effect modeled via DFT calculations as resulting from a small opening of the Fe-N-O angle. These spectral differences demonstrate different mechanisms of activation by synthetic activators, such as YC-1 and BAY 41-2272, and excess NO.
Figures


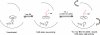
Similar articles
-
Probing soluble guanylate cyclase activation by CO and YC-1 using resonance Raman spectroscopy.Biochemistry. 2010 May 11;49(18):3815-23. doi: 10.1021/bi902214j. Biochemistry. 2010. PMID: 20353168 Free PMC article.
-
YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit.Biochemistry. 2014 Jan 14;53(1):101-14. doi: 10.1021/bi4015133. Epub 2013 Dec 30. Biochemistry. 2014. PMID: 24328155 Free PMC article.
-
A functional domain of the alpha1 subunit of soluble guanylyl cyclase is necessary for activation of the enzyme by nitric oxide and YC-1 but is not involved in heme binding.J Biol Chem. 2003 Apr 4;278(14):12590-7. doi: 10.1074/jbc.M212740200. Epub 2003 Jan 30. J Biol Chem. 2003. PMID: 12560334
-
Binding of YC-1/BAY 41-2272 to soluble guanylate cyclase: A new perspective to the mechanism of activation.Biochem Biophys Res Commun. 2010 Jul 2;397(3):375-9. doi: 10.1016/j.bbrc.2010.05.122. Epub 2010 May 27. Biochem Biophys Res Commun. 2010. PMID: 20513359 Review.
-
NO-independent, haem-dependent soluble guanylate cyclase stimulators.Handb Exp Pharmacol. 2009;(191):277-308. doi: 10.1007/978-3-540-68964-5_13. Handb Exp Pharmacol. 2009. PMID: 19089334 Review.
Cited by
-
Probing soluble guanylate cyclase activation by CO and YC-1 using resonance Raman spectroscopy.Biochemistry. 2010 May 11;49(18):3815-23. doi: 10.1021/bi902214j. Biochemistry. 2010. PMID: 20353168 Free PMC article.
-
YC-1 binding to the β subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the α subunit.Biochemistry. 2014 Jan 14;53(1):101-14. doi: 10.1021/bi4015133. Epub 2013 Dec 30. Biochemistry. 2014. PMID: 24328155 Free PMC article.
-
Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content.J Biol Chem. 2014 May 30;289(22):15259-71. doi: 10.1074/jbc.M114.559393. Epub 2014 Apr 14. J Biol Chem. 2014. PMID: 24733395 Free PMC article.
-
Heme sensor proteins.J Biol Chem. 2013 May 10;288(19):13194-203. doi: 10.1074/jbc.R112.422642. Epub 2013 Mar 28. J Biol Chem. 2013. PMID: 23539616 Free PMC article. Review.
-
Structure/function of the soluble guanylyl cyclase catalytic domain.Nitric Oxide. 2018 Jul 1;77:53-64. doi: 10.1016/j.niox.2018.04.008. Epub 2018 Apr 25. Nitric Oxide. 2018. PMID: 29702251 Free PMC article. Review.
References
-
- Butler AR, Williams DLH. The physiological role of nitric oxide. Chemical Society Review. 1993;22:233–241.
-
- Bian K, Murad F. Nitric oxide (NO) -- Biogeneration, regulation, and relevance to human diseases. Frontiers in Bioscience. 2003;8:d264–d278. - PubMed
-
- Derbyshire ER, Marletta MA. Biochemistry of soluble guanylate cyclase. In: Schmidt HHHW, Hofmann F, Stasch J-P, editors. Handbook of Experimental Pharmacology. Springer-Verlag; Berlin Heidelberg: 2009. pp. 17–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

