Silver nanosystems for photoacoustic imaging and image-guided therapy
- PMID: 20459238
- PMCID: PMC2859084
- DOI: 10.1117/1.3365937
Silver nanosystems for photoacoustic imaging and image-guided therapy
Abstract
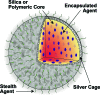
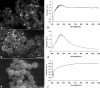
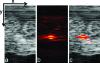
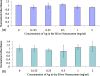
Due to their optical absorption properties, metallic nanoparticles are excellent photoacoustic imaging contrast agents. A silver nanosystem is presented here as a potential contrast agent for photoacoustic imaging and image-guided therapy. Currently, the nanosystem consists of a porous silver layer deposited on the surface of spherical silica cores ranging in diameter from 180 to 520 nm. The porous nature of the silver layer will allow for release of drugs or other therapeutic agents encapsulated in the core in future applications. In their current PEGylated form, the silver nanosystem is shown to be nontoxic in vitro at concentrations of silver up to 2 mgml. Furthermore, the near-infrared absorbance properties of the nanosystem are demonstrated by measuring strong, concentration-dependent photoacoustic signal from the silver nanosystem embedded in an ex vivo tissue sample. Our study suggests that silver nanosystems can be used as multifunctional agents capable of augmenting image-guided therapy techniques.
Figures




References
-
- Bell A. G., “Production of sound by radiant energy,” J. Franklin Inst. JFINAB 111(6), 401–426 (1881).10.1016/0016-0032(81)90005-3 - DOI
-
- Emelianov S., Aglyamov S., Shah J., Sethuraman S., Scott G., Schmitt R., Karpiouk A., Motamedi M., and Oraevsky A., “Synergy of ultrasound, elasticity, and optoacoustic imaging for improved detection and differentiation of cancerous tissue,” J. Acoust. Soc. Am. JASMAN 115, 2411 (2004).10.1121/1.1621858 - DOI
-
- Emelianov S. Y., Aglyamov S. R., Shah J., Sethuraman S., Scott W. G., Schmitt R., Motamedi M., Karpiouk A., and Oraevsky A., “Combined ultrasound, optoacoustic, and elasticity imaging,” Proc. SPIE PSISDG 5320, 101–112 (2004).10.1117/12.537155 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

