Quantitative evaluation of high-density diffuse optical tomography: in vivo resolution and mapping performance
- PMID: 20459251
- PMCID: PMC2874047
- DOI: 10.1117/1.3368999
Quantitative evaluation of high-density diffuse optical tomography: in vivo resolution and mapping performance
Abstract
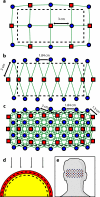
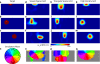
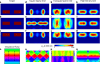
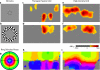
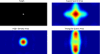
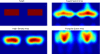
Despite the unique brain imaging capabilities and advantages of functional near-infrared spectroscopy (fNIRS), including portability and comprehensive hemodynamic measurement, widespread acceptance in the neuroimaging community has been hampered by low spatial resolution and image localization errors. While recent technical developments such as high-density diffuse optical tomography (HD-DOT) have, in principle, been shown to have superior in silico image quality, the majority of optical imaging studies are still conducted with sparse fNIRS arrays, perhaps partially because the performance increases of HD-DOT appear incremental. Without a quantitative comparative analysis between HD-DOT and fNIRS, using both simulation and in vivo neuroimaging, the implications of the new HD-DOT technology have been difficult to judge. We present a quantitative comparison of HD-DOT and two commonly used fNIRS geometries using (1) standard metrics of image quality, (2) simulated brain mapping tasks, and (3) in vivo visual cortex mapping results in adult humans. The results show that better resolution and lower positional errors are achieved with HD-DOT and that these improvements provide a substantial advancement in neuroimaging capability. In particular, we demonstrate that HD-DOT enables detailed phase-encoded retinotopic mapping, while sparse arrays are limited to imaging individual block-design visual stimuli.
Figures







References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

