Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth
- PMID: 20459265
- PMCID: PMC2874049
- DOI: 10.1117/1.3381184
Second harmonic generation imaging as a potential tool for staging pregnancy and predicting preterm birth
Erratum in
- J Biomed Opt. 2010 May-Jun;15(3):039802
Abstract
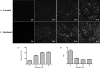
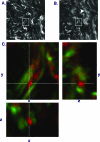
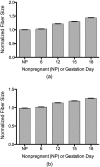
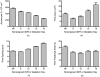
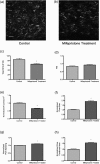
We use second harmonic generation (SHG) microscopy to assess changes in collagen structure of murine cervix during cervical remodeling of normal pregnancy and in a preterm birth model. Visual inspection of SHG images revealed substantial changes in collagen morphology throughout normal gestation. SHG images collected in both the forward and backward directions were analyzed quantitatively for changes in overall mean intensity, forward to backward intensity ratio, collagen fiber size, and porosity. Changes in mean SHG intensity and intensity ratio take place in early pregnancy, suggesting that submicroscopic changes in collagen fibril size and arrangement occur before macroscopic changes become evident. Fiber size progressively increased from early to late pregnancy, while pores between collagen fibers became larger and farther apart. Analysis of collagen features in premature cervical remodeling show that changes in collagen structure are dissimilar from normal remodeling. The ability to quantify multiple morphological features of collagen that characterize normal cervical remodeling and distinguish abnormal remodeling in preterm birth models supports future studies aimed at development of SHG endoscopic devices for clinical assessment of collagen changes during pregnancy in women and for predicting risk of preterm labor which occurs in 12.5% of all pregnancies.
Figures
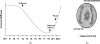

References
-
- Committee on Understanding Premature Birth and Assuring Healthy Outcomes (Ed. Board on Health Sciences Policy), National Academies Press, Washington, DC (2006).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

