Intraoperative delineation of primary brain tumors using time-resolved fluorescence spectroscopy
- PMID: 20459282
- PMCID: PMC4171753
- DOI: 10.1117/1.3374049
Intraoperative delineation of primary brain tumors using time-resolved fluorescence spectroscopy
Abstract
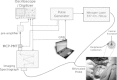
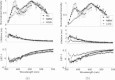
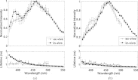
The goal of this study is to determine the potential of time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) as an adjunctive tool for delineation of brain tumor from surrounding normal tissue in order to assist the neurosurgeon in near-complete tumor excision. A time-domain TR-LIFS prototype apparatus (gated photomultiplier detection, fast digitizer) was used for recording tissue autofluorescence in normal cortex (NC), normal white matter (NWM), and various grades of gliomas intraoperatively. Tissue fluorescence was induced with a pulsed nitrogen laser (337 nm, 700 ps), and the intensity decay profiles were recorded in the 360- to 550-nm spectral range (10-nm interval). Histopathological analysis (hematoxylin & eosin) of the biopsy samples taken from the site of TR-LIFS measurements was used for validation of spectroscopic results. Preliminary results on 17 patients demonstrate that normal cortex (N=16) and normal white matter (N=3) show two peaks of fluorescence emission at 390 nm (lifetime=1.8+/-0.3 ns) and 460 nm (lifetime=0.8+/-0.1 ns). The 390-nm emission peak is absent in low-grade glioma (N=5; lifetime=1.1 ns) and reduced in high-grade glioma (N=9; lifetime=1.7+/-0.4 ns). The emission characteristics at 460 nm in all tissues correlated with the nicotinamide adenine dinucleotide fluorescence (peak: 440 to 460 nm; lifetime: 0.8 to 1.0 ns). These findings demonstrate the potential of using TR-LIFS as a tool for enhanced delineation of brain tumors during surgery. In addition, this study evaluates similarities and differences between TR-LIFS signatures of brain tumors obtained in vivo and those previously reported in ex vivo brain tumor specimens.
Figures



References
-
- Fazekas J. T., “Treatment of grades I and II brain astrocytomas. The role of radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. IOBPD3 2(7–8), 661–666 (1977). - PubMed
-
- Croce A. C., Fiorani S., Locatelli D., Nano R., Ceroni M., Tancioni F., Giombelli E., Benericetti E., and Bottiroli G., “Diagnostic potential of autofluorescence for an assisted intraoperative delineation of glioblastoma resection margins,” Photochem. Photobiol. PHCBAP 77(3), 309–318 (2003).10.1562/0031-8655(2003)077<0309:DPOAFA>2.0.CO;2 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

