Long-term effect of the Women's Health Initiative study on antiosteoporosis medication prescribing
- PMID: 20459329
- PMCID: PMC2875952
- DOI: 10.1089/jwh.2009.1441
Long-term effect of the Women's Health Initiative study on antiosteoporosis medication prescribing
Abstract
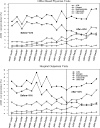
Aim: To describe long-term prescribing patterns of osteoporosis therapy before and after the Women's Health Initiative (WHI) publication.
Methods: We conducted a time-series analysis from 1997 to 2005 using nationally representative data based on office-based physician and hospital ambulatory clinic visits. Bivariate and multivariable analyses were conducted using chi-square tests and logistic regression, respectively, and trends in the prevalence of osteoporosis therapies were evaluated per 6-month (semiannual) intervals. Linear regression and graphic techniques were used to determine statistical differences in the prevalence trends between the two periods.
Results: Overall prevalence of therapeutic or preventive osteoporosis therapy was similar between the WHI periods. However, a significant decrease in estrogen therapy and increases in bisphosphonates, calcium/vitamin D were observed in the period after the WHI publication (p < 0.05). Multiple logistic regression analysis showed older age and white race were associated with a higher likelihood of antiosteoporosis medication (AOM) prescription, and Medicaid insurance type was associated with a lower likelihood of an AOM prescription. Excluding calcium/vitamin D, nonestrogen therapy was more likely to be prescribed in the after-WHI period (office-based physician clinic: [adjusted OR, aOR] 2.49 [2.04-4.04]; hospital-based clinic: aOR 2.42 [1.67-7.50]) Nonestrogen therapy was more prevalent in visits made by older women, women of white race, women with contraindicated conditions for estrogen therapy, and women from the Northeast region.
Conclusions: After the WHI publication, the overall prevalence of osteoporosis therapy did not change; however, a shift from estrogen to nonestrogen therapy was observed after the WHI publication. Black women were less likely to receive nonestrogen antiosteoporosis therapy in hospital-based clinics.
Figures
Comment in
-
Climacteric commentaries. Women's Health Initiative, osteoporosis prescribing and fracture incidence.Climacteric. 2010 Oct;13(5):502. Climacteric. 2010. PMID: 20815099 No abstract available.
References
-
- Rossouw JE. Anderson GL. Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. - PubMed
-
- Prempro® package insert. www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/ [Jul 1;2009 ]. www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/
-
- Hillman JJ. Zuckerman IH. Lee E. The impact of the Women's Health Initiative on hormone replacement therapy in a Medicaid program. J Womens Health. 2004;13:986–992. - PubMed
-
- Majumdar SR. Almasi EA. Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women's Health Initiative. JAMA. 2004;292:1983–1988. - PubMed
-
- McIntosh J. Blalock S. Effects of media coverage of Women's Health Initiative study on attitudes and behavior of women receiving hormone replacement therapy. Am J Health Syst Pharm. 2005;62:69–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

