The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum
- PMID: 20459597
- PMCID: PMC2898085
- DOI: 10.1186/gb-2010-11-5-r48
The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum
Abstract
Background: Various drugs of abuse activate intracellular pathways in the brain reward system. These pathways regulate the expression of genes that are essential to the development of addiction. To reveal genes common and distinct for different classes of drugs of abuse, we compared the effects of nicotine, ethanol, cocaine, morphine, heroin and methamphetamine on gene expression profiles in the mouse striatum.
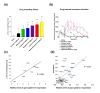
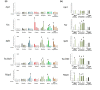
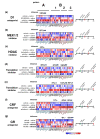
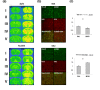
Results: We applied whole-genome microarray profiling to evaluate detailed time-courses (1, 2, 4 and 8 hours) of transcriptome alterations following acute drug administration in mice. We identified 42 drug-responsive genes that were segregated into two main transcriptional modules. The first module consisted of activity-dependent transcripts (including Fos and Npas4), which are induced by psychostimulants and opioids. The second group of genes (including Fkbp5 and S3-12), which are controlled, in part, by the release of steroid hormones, was strongly activated by ethanol and opioids. Using pharmacological tools, we were able to inhibit the induction of particular modules of drug-related genomic profiles. We selected a subset of genes for validation by in situ hybridization and quantitative PCR. We also showed that knockdown of the drug-responsive genes Sgk1 and Tsc22d3 resulted in alterations to dendritic spines in mice, possibly reflecting an altered potential for plastic changes.
Conclusions: Our study identified modules of drug-induced genes that share functional relationships. These genes may play a critical role in the early stages of addiction.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

