Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells
- PMID: 20459645
- PMCID: PMC2881115
- DOI: 10.1186/1476-4598-9-101
Osteopontin selectively regulates p70S6K/mTOR phosphorylation leading to NF-kappaB dependent AP-1-mediated ICAM-1 expression in breast cancer cells
Abstract
Background: Breast cancer is one of the most frequently diagnosed cancer and accounts for over 400,000 deaths each year worldwide. It causes premature death in women, despite progress in early detection, treatment, and advances in understanding the molecular basis of the disease. Therefore, it is important to understand the in depth mechanism of tumor progression and develop new strategies for the treatment of breast cancer. Thus, this study is aimed at gaining an insight into the molecular mechanism by which osteopontin (OPN), a member of SIBLING (Small Integrin Binding LIgand N-linked Glycoprotein) family of protein regulates tumor progression through activation of various transcription factors and expression of their downstream effector gene(s) in breast cancer.
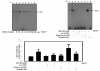
Results: In this study, we report that purified native OPN induces ICAM-1 expression in breast cancer cells. The data revealed that OPN induces NF-kappaB activation and NF-kappaB dependent ICAM-1 expression. We also observed that OPN-induced NF-kappaB further controls AP-1 transactivation, suggesting that there is cross talk between NF-kappaB and AP-1 which is unidirectional towards AP-1 that in turn regulates ICAM-1 expression in these cells. We also delineated the role of mTOR and p70S6 kinase in OPN-induced ICAM-1 expression. The study suggests that inhibition of mTOR by rapamycin augments whereas overexpression of mTOR/p70S6 kinase inhibits OPN-induced ICAM-1 expression. Moreover, overexpression of mTOR inhibits OPN-induced NF-kappaB and AP-1-DNA binding and transcriptional activity. However, rapamycin further enhanced these OPN-induced effects. We also report that OPN induces p70S6 kinase phosphorylation at Thr-421/Ser-424, but not at Thr-389 or Ser-371 and mTOR phosphorylation at Ser-2448. Overexpression of mTOR has no effect in regulation of OPN-induced phosphorylation of p70S6 kinase at Thr-421/Ser-424. Inhibition of mTOR by rapamycin attenuates Ser-371 phosphorylation but does not have any effect on Thr-389 and Thr-421/Ser-424 phosphorylation of p70S6 kinase. However, OPN-induced phosphorylation of p70S6 kinase at Thr-421/Ser-424 is being controlled by MEK/ERK pathway.
Conclusion: These results suggest that blocking of OPN-induced ICAM-1 expression through mTOR/p70S6 kinase signaling pathway may be an important therapeutic strategy for the treatment of breast cancer.
Figures






References
-
- Kammerer S, Roth RB, Reneland R, Marnellos G, Hoyal CR, Markward NJ, Ebner F, Kiechle M, Schwarz-Boeger U, Griffiths LR, Ulbrich C, Chrobok K, Forster G, Praetorius GM, Meyer P, Rehbock J, Cantor CR, Nelson MR, Braun A. Large scale association study identifies ICAM gene region as breast and prostate cancer susceptibility locus. Cancer Res. 2004;64:8906–10. doi: 10.1158/0008-5472.CAN-04-1788. - DOI - PubMed
-
- Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem. 2003;278:28593–606. doi: 10.1074/jbc.M303445200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

