Cadmium induces Wnt signaling to upregulate proliferation and survival genes in sub-confluent kidney proximal tubule cells
- PMID: 20459685
- PMCID: PMC2873433
- DOI: 10.1186/1476-4598-9-102
Cadmium induces Wnt signaling to upregulate proliferation and survival genes in sub-confluent kidney proximal tubule cells
Abstract
Background: The class 1 carcinogen cadmium (Cd2+) disrupts the E-cadherin/beta-catenin complex of epithelial adherens junctions (AJs) and causes renal cancer. Deregulation of E-cadherin adhesion and changes in Wnt/beta-catenin signaling are known to contribute to carcinogenesis.
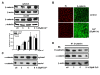
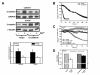
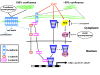
Results: We investigated Wnt signaling after Cd2+-induced E-cadherin disruption in sub-confluent cultured kidney proximal tubule cells (PTC). Cd2+ (25 microM, 3-9 h) caused nuclear translocation of beta-catenin and triggered a Wnt response measured by TOPflash reporter assays. Cd2+ reduced the interaction of beta-catenin with AJ components (E-cadherin, alpha-catenin) and increased binding to the transcription factor TCF4 of the Wnt pathway, which was upregulated and translocated to the nucleus. While Wnt target genes (c-Myc, cyclin D1 and ABCB1) were up-regulated by Cd2+, electromobility shift assays showed increased TCF4 binding to cyclin D1 and ABCB1 promoter sequences with Cd2+. Overexpression of wild-type and mutant TCF4 confirmed Cd2+-induced Wnt signaling. Wnt signaling elicited by Cd2+ was not observed in confluent non-proliferating cells, which showed increased E-cadherin expression. Overexpression of E-cadherin reduced Wnt signaling, PTC proliferation and Cd2+ toxicity. Cd2+ also induced reactive oxygen species dependent expression of the pro-apoptotic ER stress marker and Wnt suppressor CHOP/GADD153 which, however, did not abolish Wnt response and cell viability.
Conclusions: Cd2+ induces Wnt signaling in PTC. Hence, Cd2+ may facilitate carcinogenesis of PTC by promoting Wnt pathway-mediated proliferation and survival of pre-neoplastic cells.
Figures




Similar articles
-
The role of Wnt/beta-catenin signaling in renal carcinogenesis: lessons from cadmium toxicity studies.Curr Mol Med. 2010 Jun;10(4):387-404. doi: 10.2174/156652410791316986. Curr Mol Med. 2010. PMID: 20455852 Review.
-
Cadmium induces nuclear translocation of beta-catenin and increases expression of c-myc and Abcb1a in kidney proximal tubule cells.Biometals. 2007 Oct;20(5):807-20. doi: 10.1007/s10534-006-9044-9. Epub 2006 Nov 29. Biometals. 2007. PMID: 17136310
-
N-glycosylation gene DPAGT1 is a target of the Wnt/beta-catenin signaling pathway.J Biol Chem. 2010 Oct 8;285(41):31164-73. doi: 10.1074/jbc.M110.149195. Epub 2010 Aug 6. J Biol Chem. 2010. PMID: 20693288 Free PMC article.
-
p15RS attenuates Wnt/{beta}-catenin signaling by disrupting {beta}-catenin·TCF4 Interaction.J Biol Chem. 2010 Nov 5;285(45):34621-31. doi: 10.1074/jbc.M110.148791. Epub 2010 Aug 25. J Biol Chem. 2010. PMID: 20739273 Free PMC article.
-
[In situ analyses of molecular mechanisms of colorectal carcinogenesis].Pathologe. 2013 Nov;34 Suppl 2:269-73. doi: 10.1007/s00292-013-1821-y. Pathologe. 2013. PMID: 24196627 Review. German.
Cited by
-
Environmental Exposure to Heavy Metals Contributes to Diseases Via Deregulated Wnt Signaling Pathways.Iran J Pharm Res. 2021 Spring;20(2):370-382. doi: 10.22037/ijpr.2021.114897.15089. Iran J Pharm Res. 2021. PMID: 34567167 Free PMC article. Review.
-
Fetoplacental Disposition and Toxicity of Cadmium in Mice Lacking the Bcrp Transporter.Toxicol Sci. 2023 Nov 6;197(2):132-46. doi: 10.1093/toxsci/kfad115. Online ahead of print. Toxicol Sci. 2023. PMID: 37941438 Free PMC article.
-
Alternative splicing of SLC39A14 in colorectal cancer is regulated by the Wnt pathway.Mol Cell Proteomics. 2011 Jan;10(1):M110.002998. doi: 10.1074/mcp.M110.002998. Epub 2010 Oct 11. Mol Cell Proteomics. 2011. PMID: 20938052 Free PMC article.
-
Cytosolic calcium measurements in renal epithelial cells by flow cytometry.J Vis Exp. 2014 Oct 28;(92):e51857. doi: 10.3791/51857. J Vis Exp. 2014. PMID: 25407650 Free PMC article.
-
Live and Let Die: Roles of Autophagy in Cadmium Nephrotoxicity.Toxics. 2015 Apr 13;3(2):130-151. doi: 10.3390/toxics3020130. Toxics. 2015. PMID: 29056654 Free PMC article. Review.
References
-
- Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

